2025-07-08 東京科学大学
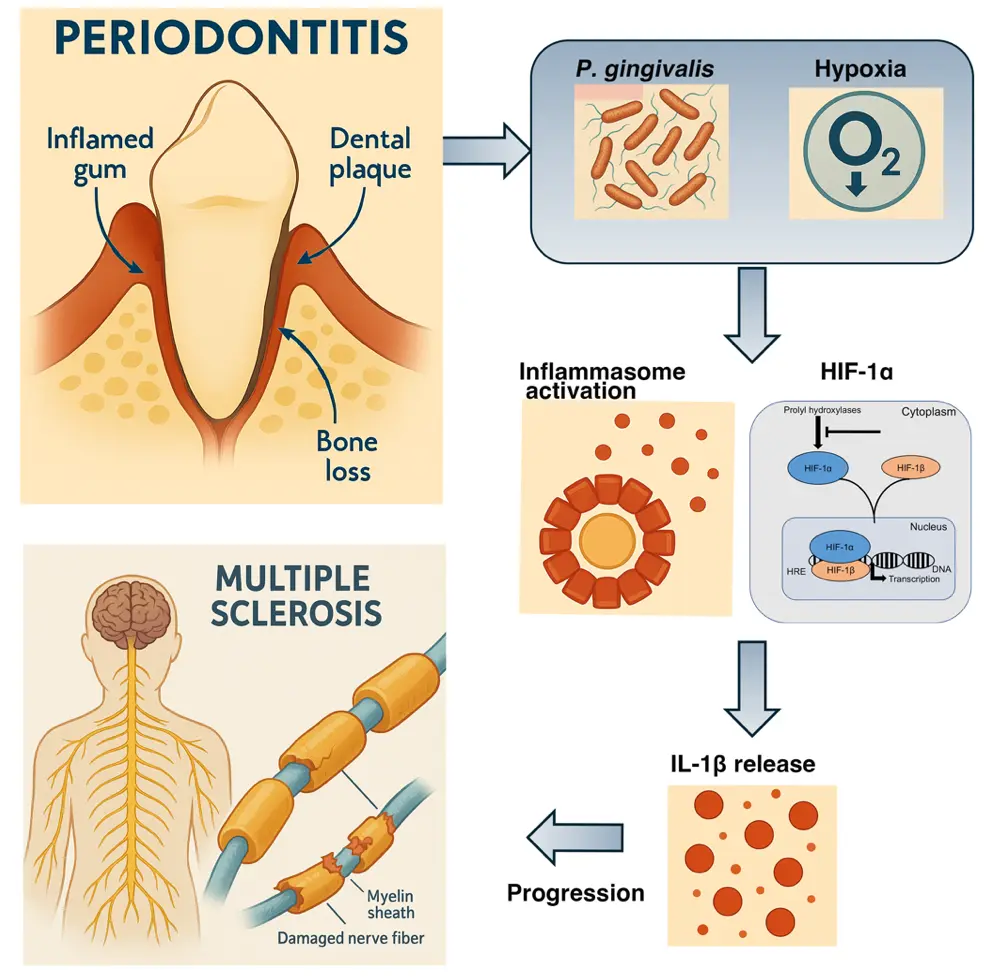
低酸素環境が歯周病原細菌 P. gingivalis によるインフラマソームの活性化を亢進し、多発性硬化症の増悪化を誘導する。
<関連情報>
- https://www.isct.ac.jp/ja/news/3r822g94rui0
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1878&prevId=&key=a0bd1de06b57f5ebb4610c3a26d900df.pdf
- https://www.nature.com/articles/s41420-025-02548-z
Hypoxia drives progression of multiple sclerosis by enhancing the inflammasome activation in macrophages with Porphyromonas gingivalis infection
Tokuju Okano,Hiroshi Ashida,Masayuki Tsukasaki,Tamako Iida,Masahiro Yamamoto,Hiroshi Takayanagi,Takeharu Sakamoto & Toshihiko Suzuki
Cell Death Discovery Published:10 June 2025
DOI:https://doi.org/10.1038/s41420-025-02548-z
Abstract
Porphyromonas gingivalis (Pg), a gram-negative anaerobic bacterium, is a leading pathogen causing periodontitis. It secretes several virulence factors, including gingipains, lipopolysaccharides (LPS), and outer membrane vesicles (OMVs), which can trigger the release of inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor alpha (TNFα), and IL-6 through inflammasome activation and Toll-like receptor (TLR) signaling. We demonstrated that Pg infection under hypoxic conditions enhances NLRP3 inflammasome activation in macrophages. Additionally, we observed that toll-interleukin-1 receptor domain-containing adaptor-inducing interferon-β (TRIF)-mediated hypoxia-inducible factor 1 alpha (HIF-1α) regulation exacerbates inflammasome activation under hypoxia. Notably, HIF-1α deficiency in myeloid cells reversed neurological symptoms and reduced IL-1β and IL-17 production in a mouse model of multiple sclerosis with Pg infection. Our findings indicated that hypoxia modulates inflammasome activation in response to periodontitis-related bacterial infections, contributing to the progression of autoimmune diseases.

