2025-07-18 東京科学大学
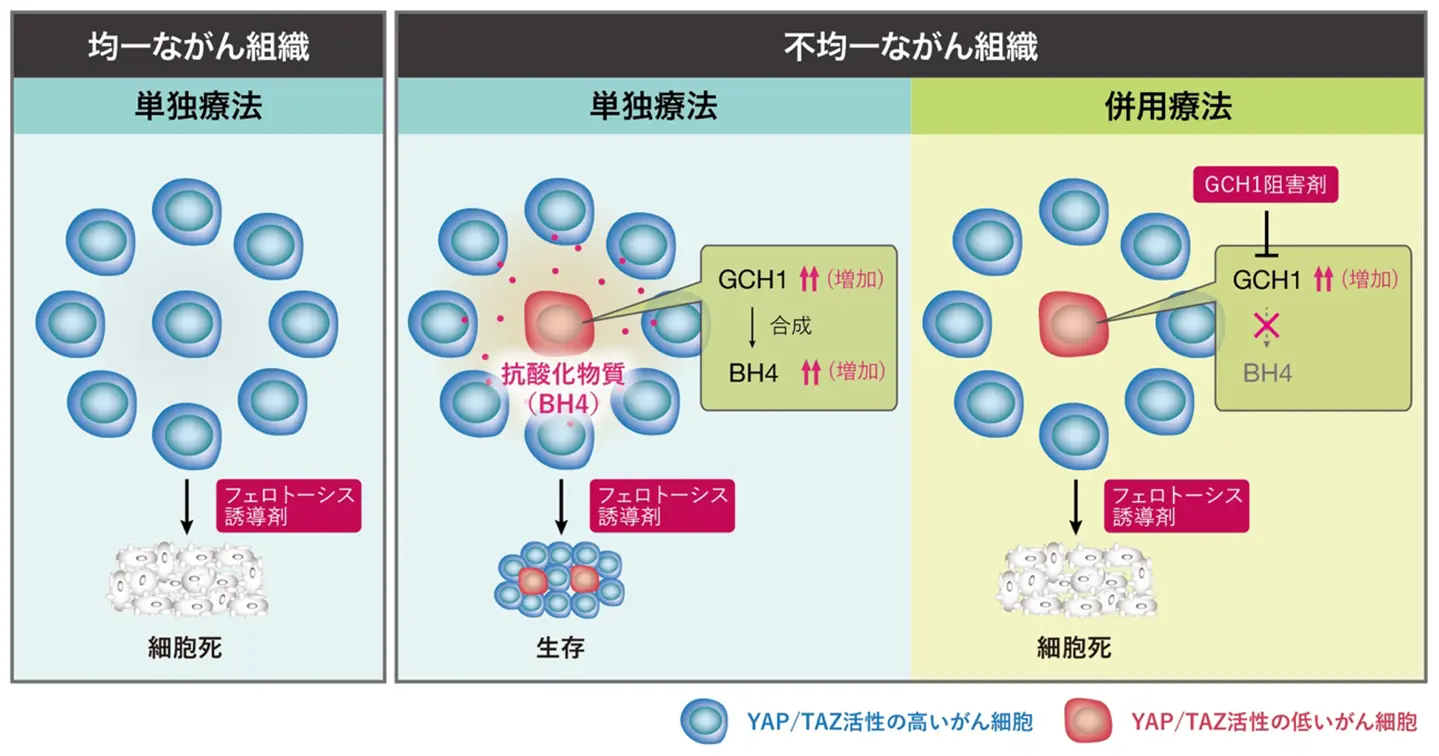 図. 肺がん細胞の“助け合い”メカニズムによる治療抵抗性の獲得と併用療法による克服
図. 肺がん細胞の“助け合い”メカニズムによる治療抵抗性の獲得と併用療法による克服
<関連情報>
- https://www.isct.ac.jp/ja/news/56o104e4i05m
- https://www.embopress.org/doi/full/10.1038/s44319-025-00515-4
ヒッポ経路はビオプテリン代謝を制御し、肺がんにおけるフェロプトーシスから隣接細胞を保護する Hippo pathway controls biopterin metabolism to shield adjacent cells from ferroptosis in lung cancer
Hao Li, Yohei Kanamori, Akihiro Nita, Ayato Maeda, Tianli Zhang, Kenta Kikuchi, Hiroyuki Yamada, Touya Toyomoto, Mohamed Fathi Saleh, Mayumi Niimura, Hironori Hinokuma, Mayuko Shimoda, Koei Ikeda, Makoto Suzuki, Yoshihiro Komohara, Daisuke Kurotaki, Tomohiro Sawa, and Toshiro Moroishi
EMBO reports Published:6 July 2025
DOI:https://doi.org/10.1038/s44319-025-00515-4
Abstract
Recent advances in single-cell technologies have uncovered significant cellular diversity in tumors, influencing cancer progression and treatment outcomes. The Hippo pathway controls cell proliferation through its downstream effectors: yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ). Our analysis of human lung adenocarcinoma and murine models revealed that cancer cells display heterogeneous YAP/TAZ activation levels within tumors. Murine lung cancer cells with high YAP/TAZ activity grow rapidly but are sensitive to ferroptosis, a cell death induced by lipid peroxidation. In contrast, cells with low YAP/TAZ activity grow slowly but resist ferroptosis. Moreover, they protect neighbouring cells from ferroptosis, creating a protective microenvironment that enhances the tumor’s resistance to ferroptosis. Mechanistically, inhibiting YAP/TAZ upregulates GTP cyclohydrolase 1 (GCH1), an enzyme critical for the biosynthesis of tetrahydrobiopterin (BH4), which functions as a secretory antioxidant to prevent lipid peroxidation. Pharmacological inhibition of GCH1 sensitizes lung cancer cells to ferroptosis inducers, suggesting a potential therapeutic approach. Our data highlights the non-cell-autonomous roles of the Hippo pathway in creating a ferroptosis-resistant tumor microenvironment.
Synopsis
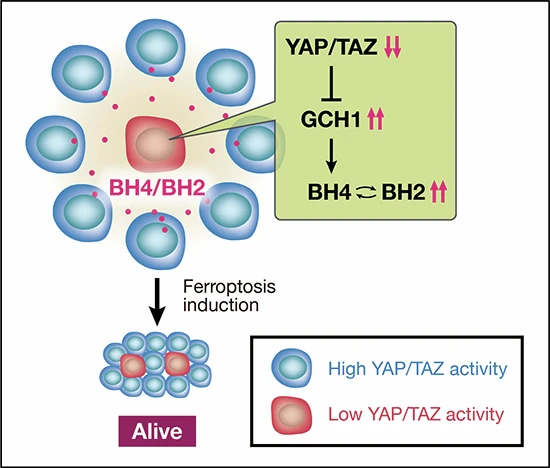
Heterogeneous Hippo pathway (YAP/TAZ) activation in lung adenocarcinoma drives tumor progression by enabling ferroptosis resistance via non-cell-autonomous mechanisms. YAP/TAZ-low cells upregulate GCH1, increasing BH4 secretion, which protects neighboring YAP/TAZ-high cells from ferroptosis.
•YAP/TAZ activity is heterogeneous in lung adenocarcinoma and correlates with poor prognosis.
•YAP/TAZ-low cells evade ferroptosis and protect nearby YAP/TAZ-high cells through BH4 secretion driven by GCH1 upregulation.
•GCH1 inhibition sensitizes tumors to ferroptosis inducers, reducing tumor growth and improving survival in a mouse model.
