2025-09-03 マウントサイナイ医療システム (MSHS)
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/obtaining-prefrontal-cortex-biopsies-during-deep-brain-stimulation-adds-no-risk-to-procedure
- https://journals.lww.com/neurosurgery/abstract/9900/safety_of_prefrontal_cortex_biopsies_during_deep.1824.aspx
深部脳刺激術中の前頭前皮質生検の安全性 Safety of Prefrontal Cortex Biopsies During Deep Brain Stimulation Procedures
Akkus, Sema MD; Simons, Nicole W. MA; Mahtani, Sansara BA; Epstein, Ethan BA; Zuccaro, Philip BSc; Hashemi, Alice BSc; Naderi, Saum MA; Ng, Klaren BA; Chong, Renata Gonzalez BA; Pierce-Scher, Skyler BA; Rogers, Henry BSc; Ona, Gerard MD; Postolovsky, Bohdana BA; Keller, Kate BA; Liharska, Lora E. PhD; Nadkarni, Girish N. MD, MPH; Charney, Alexander W. MD, PhD; Kopell, Brian H. MD
Neurosurgery Published:September 3, 2025
DOI: 10.1227/neu.0000000000003711
Abstract
BACKGROUND AND OBJECTIVES:
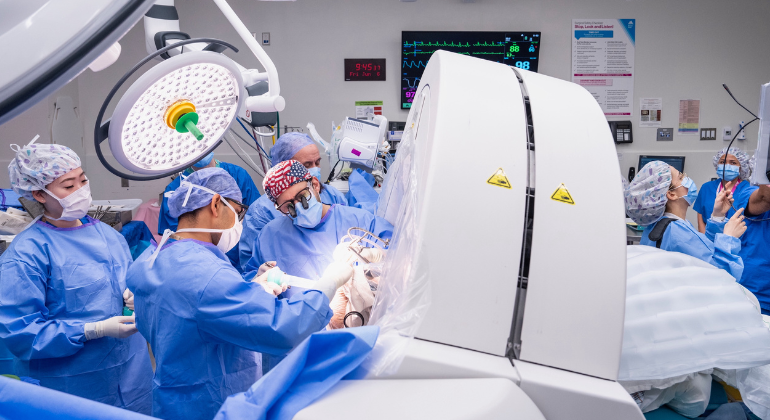
A goal of medical research is to advance knowledge of the molecular biology underlying human brain function. Yet, few studies of human brain biology have been performed using brain tissue from living people. This is due to the lack of safe approaches to sampling the living human brain for rigorous scientific inquiry. The Living Brain Project (LBP) developed a method to biopsy a small volume of prefrontal cortex (PFC) tissue during deep brain stimulation (DBS) lead placement procedures. The objective of this LBP report was to establish the safety of the PFC biopsy approach.
METHODS:
Acute adverse events (ie, infection, intracranial hemorrhage [ICH], and seizures) were tracked after 1152 DBS procedures performed on 590 patients. A PFC biopsy was obtained in 652 procedures (“biopsy group”), and no biopsy was obtained in 500 procedures (“nonbiopsy group”). Cognitive health was assessed at baseline and 1 year after DBS surgery for 144 patients. Rates of acute adverse events and changes in cognitive health were compared between the biopsy and nonbiopsy groups.
RESULTS:
No infections occurred in either group. No statistically significant difference in ICH rate was observed between groups (1.7% biopsy group vs 1.4% nonbiopsy group; χ2 test P-value = .88), and this observation held regardless of the anatomical location or the clinical severity of the ICH. No statistically significant difference in seizure rate was observed between groups (0.2% biopsy group vs 0.4% nonbiopsy group; P-value = .82). No statistically significant associations were observed between number of biopsies and changes in cognitive health over time.
CONCLUSION:
DBS procedures involving PFC biopsies for the LBP demonstrate a safety profile comparable with DBS procedures without biopsies.