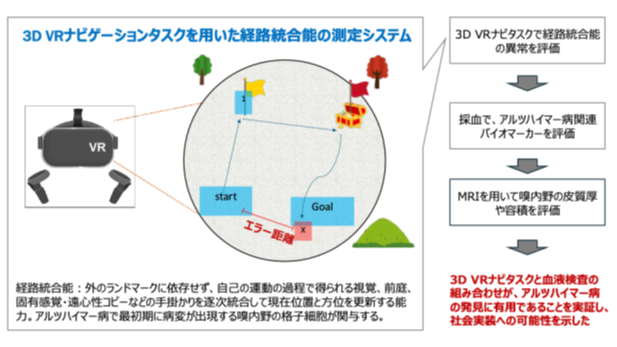
2025-09-10 岡山大学,東京大学,慶應義塾大学,国際医療福祉大学

<関連情報>
- https://www.okayama-u.ac.jp/tp/release/release_id1434.html
- https://www.okayama-u.ac.jp/up_load_files/press_r7/press20250910-1.pdf
- https://www.nature.com/articles/s44161-025-00698-y
心不全特異的心臓線維芽細胞がMYC–CXCL1–CXCR2軸を介した心機能障害に寄与 Heart failure-specific cardiac fibroblasts contribute to cardiac dysfunction via the MYC–CXCL1–CXCR2 axis
Jin Komuro,Hisayuki Hashimoto,Toshiomi Katsuki,Dai Kusumoto,Manami Katoh,Toshiyuki Ko,Masamichi Ito,Mikako Katagiri,Masayuki Kubota,Shintaro Yamada,Takahiro Nakamura,Yohei Akiba,Thukaa Kouka,Kaoruko Komuro,Mai Kimura,Shogo Ito,Seitaro Nomura,Issei Komuro,Keiichi Fukuda,Shinsuke Yuasa & Masaki Ieda
Nature Cardiovascular Research Published:10 September 2025
DOI:https://doi.org/10.1038/s44161-025-00698-y
Abstract
Heart failure (HF) is a growing global health issue. While most studies focus on cardiomyocytes, here we highlight the role of cardiac fibroblasts (CFs) in HF. Single-cell RNA sequencing of mouse hearts under pressure overload identified six CF subclusters, with one specific to the HF stage. This HF-specific CF population highly expresses the transcription factor Myc. Deleting Myc in CFs improves cardiac function without reducing fibrosis. MYC directly regulates the expression of the chemokine CXCL1, which is elevated in HF-specific CFs and downregulated in Myc-deficient CFs. The CXCL1 receptor, CXCR2, is expressed in cardiomyocytes, and blocking the CXCL1–CXCR2 axis mitigates HF. CXCL1 impairs contractility in neonatal rat and human iPSC-derived cardiomyocytes. Human CFs from failing hearts also express MYC and CXCL1, unlike those from controls. These findings reveal that HF-specific CFs contribute to HF via the MYC–CXCL1–CXCR2 pathway, offering a promising therapeutic target beyond cardiomyocytes.