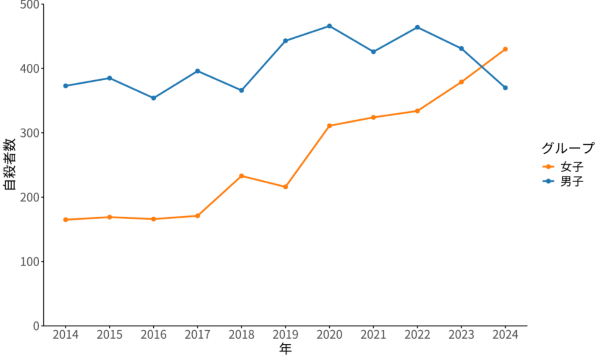
2025-09-30 国立がん研究センター,兵庫医科大学,日本臨床腫瘍研究グループ
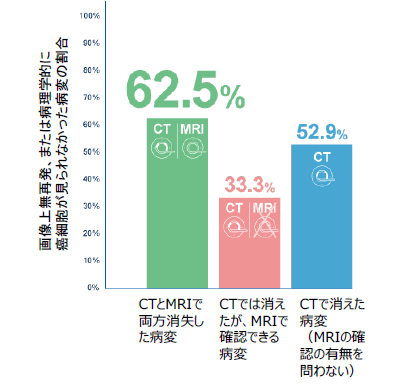
図1:画像別の消失病変と術後診断との一致率
<関連情報>
- https://www.ncc.go.jp/jp/information/pr_release/2025/0930/index.html
- https://www.ncc.go.jp/jp/information/pr_release/2025/0930/20250930.pdf
- https://jamanetwork.com/journals/jamasurgery/article-abstract/2839125
消失期大腸癌肝転移の生存不能性を評価するための画像診断の精度 Diagnostic Accuracy of Imaging in Assessing Nonviability of Disappearing Colorectal Liver Metastasis
Kozo Kataoka, MD, PhD; Murielle Mauer, PhD; Manabu Shiozawa, MD, PhD;et al
JAMA Surgery Published:September 17, 2025
DOI:10.1001/jamasurg.2025.3600
Key Points
Question How accurately can computed tomography (CT) and magnetic resonance imaging (MRI) assess the nonviability of disappearing liver metastases (DLMs) after neoadjuvant chemotherapy in patients with colorectal liver metastases (CLMs)?
Findings In this diagnostic study including 112 patients with initially unresectable CLMs, the negative predictive value of confirmed DLMs (disappeared on both CT and MRI) was below the prespecified threshold. Survival outcomes were not significantly improved with resection of confirmed DLMs.
Meaning The findings suggest that MRI may improve the accuracy in nonviable DLM detection; however, confirmed DLMs did not reliably indicate nonviability, and survival benefit of removal of cDLMs is still unclear in this setting.
Abstract
Importance In patients with colorectal liver metastases (CLMs), the optimal treatment of disappearing liver metastases (DLMs) diagnosed on postchemotherapy computed tomography (CT) is controversial.
Objective To examine the diagnostic value of magnetic resonance imaging (MRI) (diffusion weighted, T1/T2, and contrast enhanced) and CT for accurate assessment of the nonviability of DLMs.
Design, Setting, and Participants This was a prospective international study including patients with initially unresectable CLMs downstaged to liver resection after chemotherapy at 21 centers in France, Austria, Belgium, the US, and Japan. A total of 233 patients were registered and 112 were enrolled between November 2016 and March 2021 with a minimum 2-year follow-up. Clinical cutoff was in September 2023, and data were analyzed from August 2024 to May 2025.
Exposures Postchemotherapy evaluation with both CT and MRI was performed. DLMs were defined as lesions that had disappeared on CT. Confirmed DLMs (cDLMs) were defined as those that had disappeared on both CT and MRI.
Main Outcomes and Measures The primary end point was the negative predictive value (NPV) of MRI and CT in assessing the nonviability of cDLMs using either pathological complete response (for resected lesions) or the absence of recurrence at the site of cDLMs during the 2-year follow-up (for lesions left behind) to confirm the true lesion status. The planned sample size was 149 evaluable cDLMs, aiming at excluding an NPV of 0.85 or lower with a 1-sided α of 5% and a power of 90%.
Results Among 112 total patients (mean [SD] age, 60.0 [10.4] years; 67 [59.8%] male) a total of 152 cDLMs and 227 DLMs were evaluable. The NPV of all evaluable cDLMs, either resected or left behind, was 62.5% (95/152; 90% CI,50.8-74.2), which was lower than the prespecified threshold. The NPV of DLMs was 52.9%. The NPVs of resected cDLMs vs those left behind were 56.8% (50/88; 90% CI, 44.2-69.5) and 70.3% (45/64; 90% CI, 48.6-92.0), respectively. For patients without extrahepatic metastases who had R0/1 resection, there was no significant difference in disease-free survival and overall survival between those with all cDLMs removed vs those with at least 1 cDLM left behind.
Conclusions and Relevance Although the combination of MRI and CT was more accurate in detection of nonviable DLMs compared to CT alone, cDLMs did not correspond to nonviability in patients with initially unresectable CLM. Survival benefit associated with removal of cDLMs is still unclear in this setting.
Trial Registration ClinicalTrials.gov Identifier: NCT02781935