2025-10-08 カリフォルニア大学リバーサイド校(UCR)
Web要約 の発言:
<関連情報>
- https://news.ucr.edu/articles/2025/10/08/how-brain-myelin-damage-could-lead-seizures-ms
- https://www.sciencedirect.com/science/article/pii/S0969996125003420?via%3Dihub#ac0005
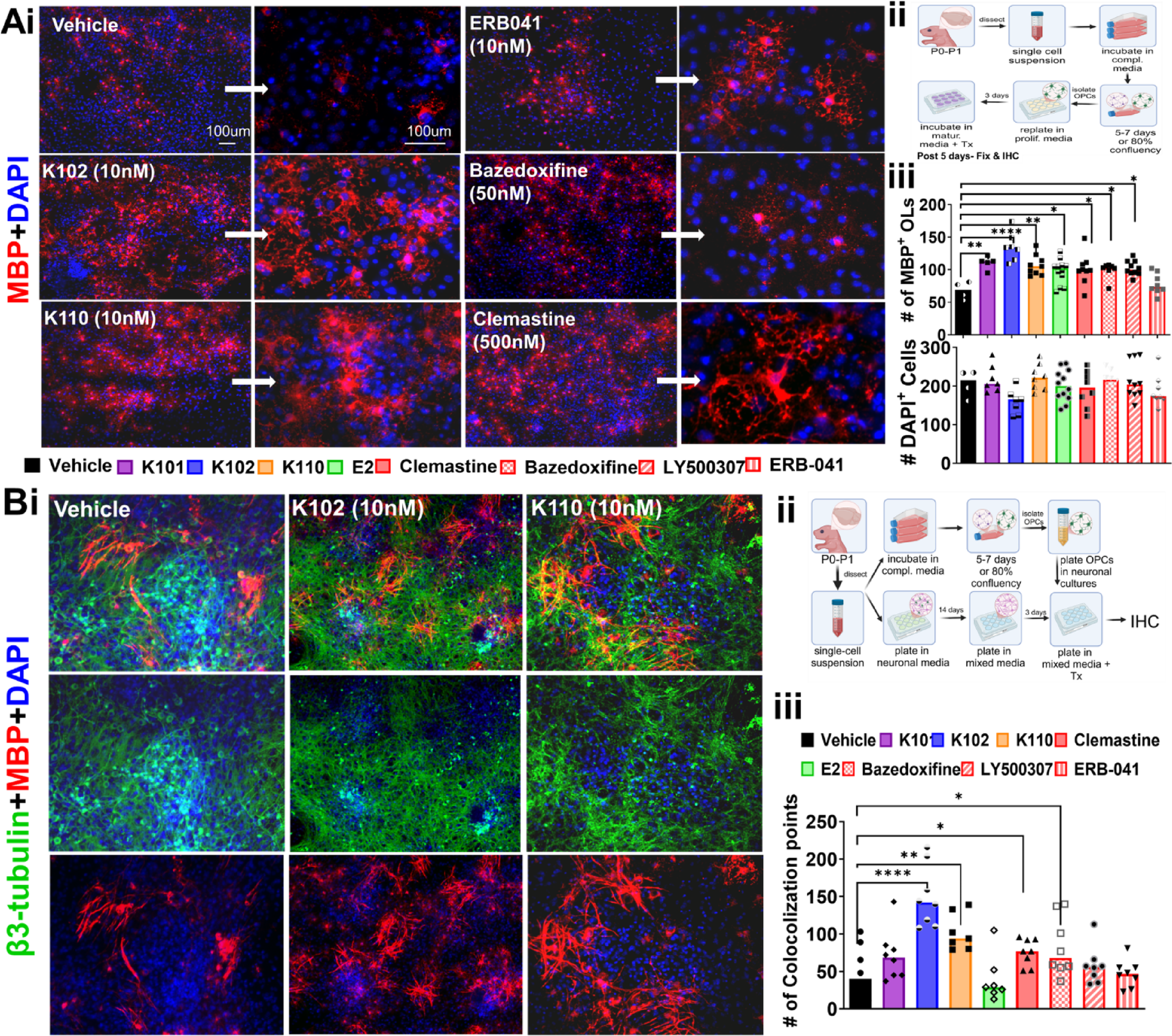
脱髄誘発性グルタミン酸不均衡が海馬の過興奮を媒介する Demyelination-induced glutamatergic imbalance mediates hippocampal Hyperexcitability
Alyssa M. Anderson, Moyinoluwa Ajayi, Carrie R. Jonak, Shane Desfor, Joselyn Soto, Adrian Akhuetie, Devang Deshpande, Andrew Lapato, Devin K. Binder, Seema K. Tiwari-Woodruff
Neurobiology of Disease Available online: 24 September 2025
DOI:https://doi.org/10.1016/j.nbd.2025.107125

Highlights
- Progressive demyelination in CPZ mice increased seizures from 38% to 88%, linking hippocampal loss to epileptogenesis.
- Chronic CA1 demyelination caused dendritic shrinkage, PV interneuron loss, and structural degeneration of pyramidal layers.
- Glutamate rose by 3 weeks and stayed elevated at excitotoxic levels, indicating sustained neurotransmitter imbalance.
- Astrocyte reactivity with GLT-1/GLAST loss and AQP4 changes impaired glutamate clearance, worsening hippocampal stress.
- Transcriptomics revealed broad neuroglial gene loss at 6 weeks with partial recovery, mapping pathways for MS epilepsy.
Abstract
Chronic demyelination is a hallmark of multiple sclerosis (MS) and is associated with increased seizure susceptibility. In this study, we used the cuprizone (CPZ) diet induced demyelination model to investigate the progression of hippocampal demyelination and its impact on seizure activity and neurotransmitter dysregulation. Using EEG recordings, immunohistochemistry, Western blotting, ELISA, Golgi staining, and NanoString transcriptomics, we found progressive hippocampal demyelination accompanied by a striking increase in seizure incidence, from 38 % at 6 weeks to 88 % by 12 weeks. Structural degeneration of the CA1 pyramidal layer was marked by reduced dendritic arborization and loss of parvalbumin interneurons. Hippocampal glutamate levels increased as early as 3 weeks and remained elevated, with values (∼2.2 μM) reaching excitotoxic thresholds, along with astrocyte reactivity (glial fibrillary acidic protein) and downregulation of astrocytic glutamate transporter-1, and glutamate aspartate Transporter-1 and modification of aquaporin-4 in CA1. Stratum pyramidal and stratum radiatum region-specific alterations in glutamate transporters and related enzymes (glutamine synthetase, glutamic acid decarboxylase 67, vesicular glutamate transporter 1), further supported neurotransmitter imbalance. Transcriptomic profiling revealed widespread downregulation of myelin, neuronal, astrocytic, glutamatergic, and GABAergic genes at 6 weeks, with partial recovery by 12 weeks. Together, these findings establish a mechanistic link between chronic hippocampal demyelination, glutamate dysregulation, and epileptogenesis offering potential molecular targets for therapeutic intervention in MS-associated epilepsy.