2025-10-13 ワシントン大学セントルイス校
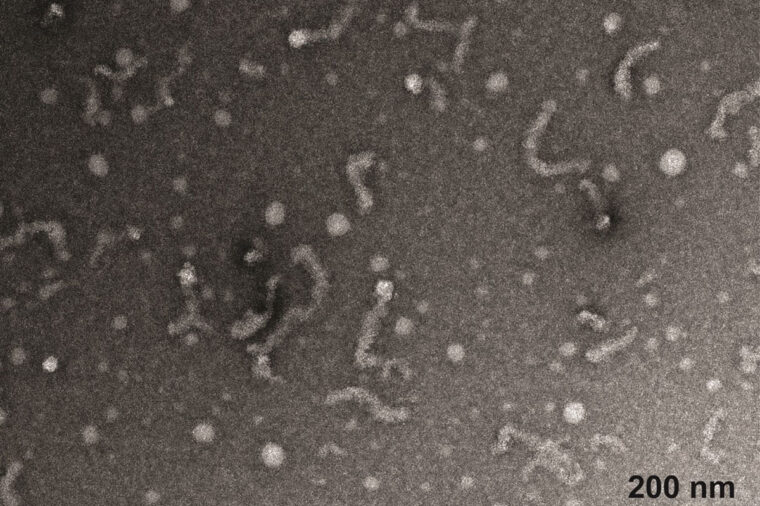
Electron microscopy of spherical and wormlike Matrin-3 assemblies. (Image: Meredith Jackrel)
<関連情報>
- https://source.washu.edu/2025/10/washu-chemists-reveal-new-insights-into-als-linked-protein/
- https://artsci.washu.edu/ampersand/washu-chemists-reveal-new-insights-als-linked-protein-meredith-jackrel
- https://www.cell.com/molecular-cell/fulltext/S1097-2765(25)00740-3
マトリン3は、RNA結合とALS/FTD関連変異によって調節される球状および虫状の集合体を形成する Matrin-3 forms spherical and wormlike assemblies that are modulated by RNA binding and ALS/FTD-associated mutations
Macy L. Sprunger ∙ Min Kyung Shinn ∙ Sabrina K. Talir ∙ Ken Lee ∙ Rohit V. Pappu ∙ Meredith E. Jackrel
Molecular Cell Published:September 19, 2025
DOI:https://doi.org/10.1016/j.molcel.2025.08.034
Highlights
- MATR3 forms nanoscale spherical and wormlike assemblies
- MATR3 assemblies undergo reversible concentration-dependent transitions
- MATR3 is a flexible inverse bolaamphiphile
- MATR3 assemblies are modulated by RNA binding and ALS-associated mutations
Summary
Matrin-3 (MATR3) is an RNA-binding protein (RBP) that is associated with familial amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). MATR3 features two RNA recognition motifs, two zinc-finger motifs, and four intrinsically disordered regions. Here, we report that human MATR3 associates with itself to form nanoscale spherical assemblies at ultralow protein concentrations. Through concentration-dependent associations, the spheres, which are 20–30 nm in diameter, transition into wormlike assemblies. These observations are reminiscent of sphere-to-worm transitions and micellization of amphiphilic molecules. Using computations and experiments, we discovered that the pattern of inter-domain attractions and repulsions gives MATR3 an inverse bolaamphiphile-like architecture that explains the concentration-dependent assembly characteristics. RNA binding causes shortening of wormlike assemblies of MATR3, whereas ALS/FTD-associated mutations render MATR3 assemblies less responsive to modulation by RNA. Overall, our findings highlight the unique assemblies formed by MATR3 while also showing how RNA-dependent interactions and ALS/FTD-associated mutations modulate the assemblies.