2025-11-05 九州大学
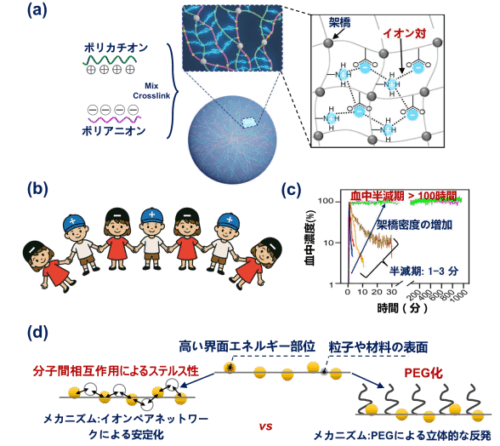
図 1. ステルス・イオンペアネットワークはどのように調製され、どのように機能するのか? (a) ポリカチオンとポリアニオンを混合し、架橋することで安定なイオンペア・ネットワークを形成する模式図。(b) イオンペア・ネットワーク・クロークの模式的なイラスト。(c) 架橋度の異なるイオンペア・ネットワークで被覆したナノマシンの血中濃度の時間依存的変化。(d) イオンペア・ネットワーク・クロークと従来の PEG クロークとの比較図。
<関連情報>
- https://www.kyushu-u.ac.jp/ja/researches/view/1357
- https://www.kyushu-u.ac.jp/f/63845/25_1105_01.pdf
- https://www.nature.com/articles/s41551-025-01534-1
立体安定化に依存しないステルスクロークが難治性癌に対するナノリアクターを介した飢餓療法を可能にする Steric stabilization-independent stealth cloak enables nanoreactors-mediated starvation therapy against refractory cancer
Junjie Li,Kazuko Toh,Panyue Wen,Xueying Liu,Anjaneyulu Dirisala,Haochen Guo,Joachim F. R. Van Guyse,Saed Abbasi,Yasutaka Anraku,Yuki Mochida,Hiroaki Kinoh,Horacio Cabral,Masaru Tanaka & Kazunori Kataoka
Nature Biomedical Engineering Published:31 October 2025
DOI:https://doi.org/10.1038/s41551-025-01534-1
Abstract
The high interfacial energy of nanomaterials limits their certain biomedical applications that require stealthiness to minimize non-specific interaction with biological components. While steric repulsion-based entropic stabilization—such as PEGylation—has long been the dominant strategy for designing stealth nanomaterials, its inherent softness and susceptibility to dynamic deformation and external forces often result in only moderate stealth performance. Here we report a distinct approach to achieving stealthiness by harnessing an ion-pair network, rather than maximizing steric repulsion. Using model polyion complex nanoparticles composed of equimolar charge ratios of polycations and polyanions, we demonstrate that increasing crosslinks between the constituent polyions beyond a critical threshold effectively reduces protein adsorption and macrophage uptake, enabling prolonged circulation with a half-life exceeding 100 hours. Building on this, we develop an asparaginase-loaded vesicular nanoreactor enveloped by a semi-permeable ion-pair network sheath for asparagine starvation therapy. The extended circulation of these nanoreactors enables sustained depletion of asparagine, leading to improved therapeutic outcomes for metastatic breast and pancreatic cancers. Our findings open an avenue for improving the pharmacokinetics of nanomaterials for therapeutic delivery through delicately engineering stable intermolecular structures with holistic cooperativity.