2025-11-24 カリフォルニア大学ロサンゼルス校(UCLA)
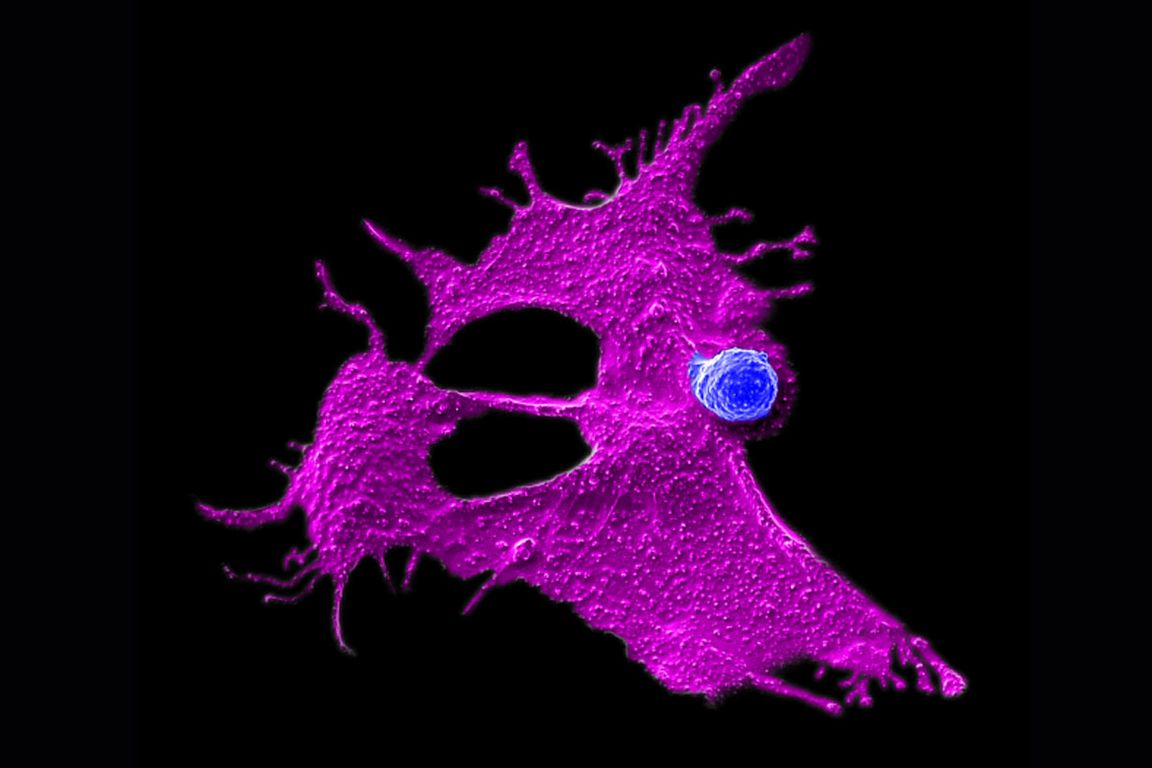
Microscopy image showing a stem cell-engineered CAR-NKT cell (blue) attacking a human solid tumor cell (magenta).
<関連情報>
- https://newsroom.ucla.edu/releases/immunotherapy-car-nkt-pancreatic-cancer-ucla
- https://www.pnas.org/doi/10.1073/pnas.2517786122
同種幹細胞工学メソテリンリダイレクトCAR-NKT細胞による同所性および転移性膵臓癌の標的化 Targeting orthotopic and metastatic pancreatic cancer with allogeneic stem cell–engineered mesothelin-redirected CAR-NKT cells
Yan-Ruide Li, Xinyuan Shen, Enbo Zhu, +5 , and Lili Yang
Proceedings of the National Academy of Sciences Published:November 21, 2025
DOI:https://doi.org/10.1073/pnas.2517786122
Significance
Pancreatic cancer (PC) is one of the deadliest cancers, often diagnosed at advanced, hard-to-treat stages. Current cell-based therapies like CAR-T cells face major hurdles, including tumor variability, immune escape, and limited scalability. In this study, we developed an off-the-shelf immunotherapy using gene-engineered natural killer T cells derived from stem cells—called Allo15MCAR-NKT cells. These cells target pancreatic tumors through multiple killing mechanisms, resist immune exhaustion, and avoid rejection by the patient’s immune system. In preclinical models, they effectively controlled tumor growth and spread. This work offers a promising step toward scalable, next-generation immunotherapy for PC, with the potential to address current treatment limitations and improve outcomes for patients with advanced disease.
Abstract
Pancreatic cancer (PC) remains one of the leading causes of cancer-related mortality worldwide. The majority of patients are diagnosed at advanced stages, with over 50% presenting with metastatic disease at the time of diagnosis. Although chimeric antigen receptor (CAR)-T cell therapy has shown promise in targeting PC, its clinical efficacy remains limited due to several critical challenges. These include tumor antigen heterogeneity, antigen loss or escape mechanisms, functional exhaustion of CAR-T cells within the tumor microenvironment, as well as inherent limitations of autologous approaches such as high manufacturing costs, prolonged production timelines, and restricted scalability. To address these challenges, we developed allogeneic IL-15–enhanced, mesothelin-specific CAR-engineered invariant natural killer T (Allo15MCAR-NKT) cells through gene engineering of human hematopoietic stem and progenitor cells (HSPCs) using a clinically guided culture method. These Allo15MCAR-NKT cells exhibited robust and multifaceted antitumor activity against PC, driven by both CAR and NK receptor–mediated cytotoxic mechanisms. In orthotopic and metastatic human PC xenograft models, Allo15MCAR-NKT cells demonstrated superior tumor control, enhanced trafficking and infiltration into tumor sites, sustained effector and cytotoxic phenotypes, and reduced expression of exhaustion markers. Importantly, Allo15MCAR-NKT cells demonstrated a favorable safety profile, characterized by the absence of graft-versus-host disease and minimal cytokine release syndrome. Collectively, these findings validate Allo15MCAR-NKT cells as a promising next-generation, off-the-shelf immunotherapeutic approach for PC, with the potential to overcome critical challenges including tumor heterogeneity, immune evasion, and therapeutic resistance, especially in the context of metastatic disease.