2026-01-07 アメリカ国立衛生研究所(NIH)
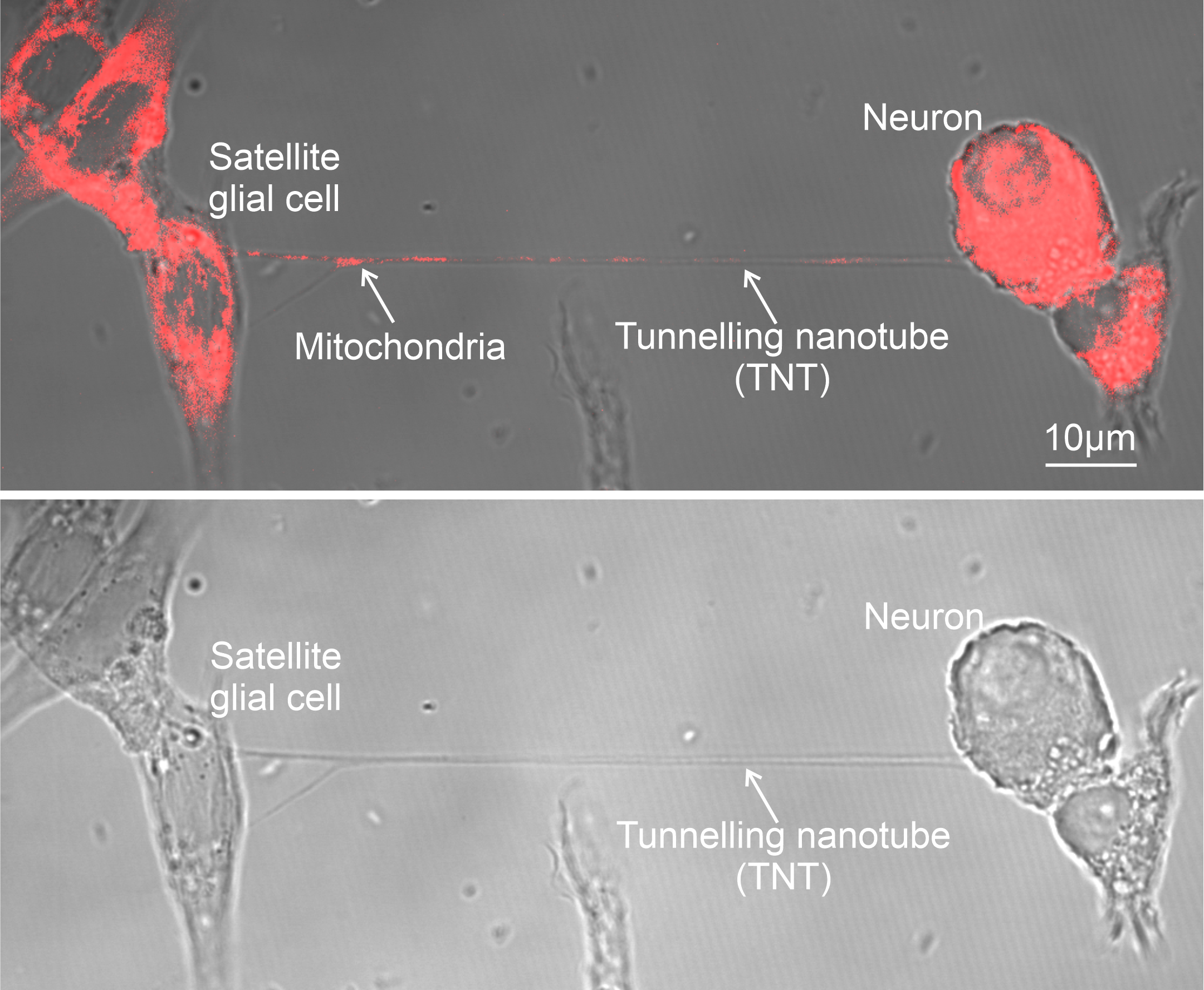 Fluorescence microscopy images show satellite glial cells transferring mitochondria (red) to neurons through nanotubes that connect the two cells.Xu et al.
Fluorescence microscopy images show satellite glial cells transferring mitochondria (red) to neurons through nanotubes that connect the two cells.Xu et al.
<関連情報>
- https://www.nih.gov/news-events/news-releases/fresh-energy-supply-may-shield-nerves-diabetic-or-chemo-induced-neuropathy
- https://www.nature.com/articles/s41586-025-09896-x
- https://www.cell.com/cell/fulltext/S0092-8674(21)01054-0
グリアからニューロンへのミトコンドリアの移動は末梢神経障害を予防する Mitochondrial transfer from glia to neurons protects against peripheral neuropathy
Jing Xu,Yize Li,Charles Novak,Min Lee,Zihan Yan,Sangsu Bang,Aidan McGinnis,Sharat Chandra,Vivian Zhang,Wei He,Terry Lechler,Maria Pia Rodriguez Salazar,Cagla Eroglu,Matthew L. Becker,Dmitry Velmeshev,Richard E. Cheney & Ru-Rong Ji
Nature Published:07 January 2026
DOI:https://doi.org/10.1038/s41586-025-09896-x
Abstract
Primary sensory neurons in dorsal root ganglia (DRG) have long axons and a high demand for mitochondria, and mitochondrial dysfunction has been implicated in peripheral neuropathy after diabetes and chemotherapy1,2. However, the mechanisms by which primary sensory neurons maintain their mitochondrial supply remain unclear. Satellite glial cells (SGCs) in DRG encircle sensory neurons and regulate neuronal activity and pain3. Here we show that SGCs are capable of transferring mitochondria to DRG sensory neurons in vitro, ex vivo and in vivo by the formation of tunnelling nanotubes with SGC-derived myosin 10 (MYO10). Scanning and transmission electron microscopy revealed tunnelling nanotube-like ultrastructures between SGCs and sensory neurons in mouse and human DRG. Blockade of mitochondrial transfer in naive mice leads to nerve degeneration and neuropathic pain. Single-nucleus RNA sequencing and in situ hybridization revealed that MYO10 is highly expressed in human SGCs. Furthermore, SGCs from DRG of people with diabetes exhibit reduced MYO10 expression and mitochondrial transfer to neurons. Adoptive transfer of human SGCs into the mouse DRG provides MYO10-dependent protection against peripheral neuropathy. This study uncovers a previously unrecognized role of peripheral glia and provides insights into small fibre neuropathy in diabetes, offering new therapeutic strategies for the management of neuropathic pain.
ミクログリアは、トンネルナノチューブを介した分配によって線維状α-シヌクレイン貨物を共同で分解する Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes
Hannah Scheiblich ∙ Cira Dansokho ∙ Dilek Mercan ∙ … ∙ Eicke Latz ∙ Ronald Melki ∙ Michael T. Heneka
Cell Published:September 22, 2021
DOI:https://doi.org/10.1016/j.cell.2021.09.007
Highlights
- Microglia rapidly engulf exogenous α-synuclein but hesitate in its degradation
- α-synuclein is transferred between microglia through tunneling nanotubes
- Healthy microglia donate mitochondria to α-synuclein overloaded cells
- Sharing the α-synuclein burden attenuated the inflammatory microglia profile
Summary
Microglia are the CNS resident immune cells that react to misfolded proteins through pattern recognition receptor ligation and activation of inflammatory pathways. Here, we studied how microglia handle and cope with α-synuclein (α-syn) fibrils and their clearance. We found that microglia exposed to α-syn establish a cellular network through the formation of F-actin-dependent intercellular connections, which transfer α-syn from overloaded microglia to neighboring naive microglia where the α-syn cargo got rapidly and effectively degraded. Lowering the α-syn burden attenuated the inflammatory profile of microglia and improved their survival. This degradation strategy was compromised in cells carrying the LRRK2 G2019S mutation. We confirmed the intercellular transfer of α-syn assemblies in microglia using organotypic slice cultures, 2-photon microscopy, and neuropathology of patients. Together, these data identify a mechanism by which microglia create an “on-demand” functional network in order to improve pathogenic α-syn clearance.