2026-01-10 京都大学高等研究院
<関連情報>
- https://ashbi.kyoto-u.ac.jp/ja/news_research/21576/
- https://www.cell.com/current-biology/fulltext/S0960-9822(25)01688-4
嫌悪条件下での動機づけは霊長類の線条体淡蒼球経路によって制御される Motivation under aversive conditions is regulated by a striatopallidal pathway in primates
Jung-min N. OH ∙ Satoko Amemori ∙ Ken-ichi Inoue ∙ Kei Kimura ∙ Masahiko Takada ∙ Ken-ichi Amemori
Current Biology Published:January 9, 2026
DOI:https://doi.org/10.1016/j.cub.2025.12.035
Highlights
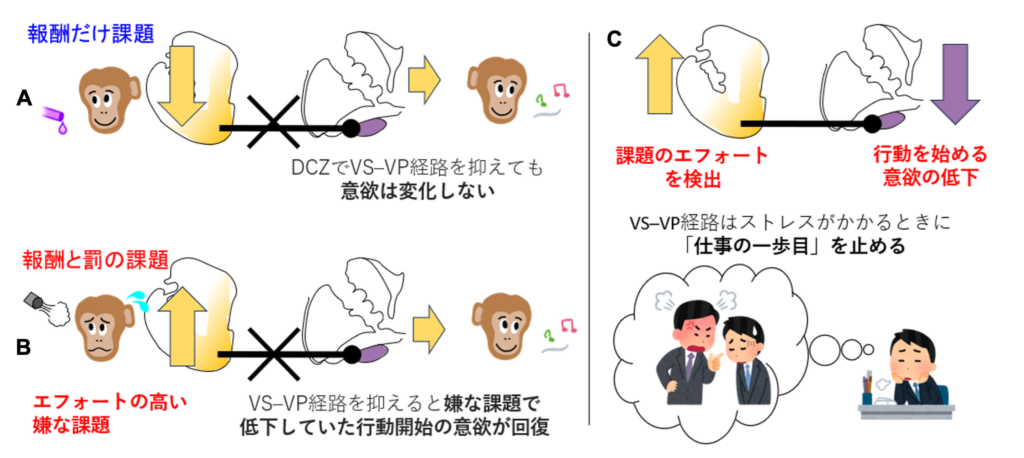
- Chemogenetic suppression of the VS-VP pathway restores motivation under aversion
- VS-VP pathway detects aversive demand and suppresses initiation of trials
- VS-VP pathway regulates trial initiation independently of goal valuation
- VS and VP neurons exhibit opposing activity patterns across motivational contexts
Summary
Motivation often diminishes under aversive conditions. Clinically, motivational deficits are linked to psychiatric disorders such as depression and schizophrenia, yet the neural mechanisms by which aversive contexts suppress motivation remain unclear. Although classical theories associate motivation with the expected value of outcomes, less is known about the neural circuits that govern effort-based behavioral initiation. To address this, we dissociated motivational drive from goal valuation using an approach-avoidance (Ap-Av) task, in which macaques evaluated outcomes combining reward and punishment (air puffs to the face). As a control, we employed an approach-approach (Ap-Ap) task based solely on reward. Using chemogenetic manipulation, we found that selective inhibition of the ventral striatum to ventral pallidum (VS-VP) pathway restored the motivation to initiate trials in the Ap-Av task without affecting goal valuation. No effects were observed in the Ap-Ap task. These findings provide causal evidence that the VS-VP pathway mediates motivational suppression in aversive contexts. Electrophysiological recordings revealed rapid VS responses to aversive cues and a gradual decrease in VP activity, suggesting an inhibitory interaction in which elevated VS activity dampens VP output to limit initiation. The slower VP dynamics may reflect a process by which aversive signals are gradually integrated to influence the motivational state. Together, these results identify the VS-VP pathway as a key circuit by which aversive contexts suppress effort-based behavioral initiation, highlighting it as a potential target for treating motivational deficits in depression and schizophrenia.


