2026-02-02 中国科学院(CAS)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202601/t20260129_1147583.shtml
- https://www.nature.com/articles/s41586-025-10029-7
Ostα/βの構造は、独特な折り畳み構造と胆汁酸輸送機構を明らかにする Structures of Ostα/β reveal a unique fold and bile acid transport mechanism
Xuemei Yang,Nana Cui,Tianyu Li,Xinheng He,Heng Zhang,Canrong Wu,Yang Li,Xiong Ma & H. Eric Xu
Nature Published:28 January 2026
DOI:https://doi.org/10.1038/s41586-025-10029-7
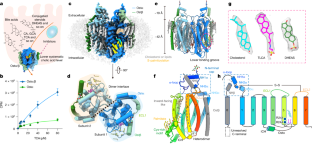
Abstract
Bile acid and steroid hormone homeostasis are critical for human health, with disruptions linked to metabolic and endocrine disorders1,2. The organic solute transporter Ostα/β, essential for bile acid efflux in enterohepatic circulation3, has long defied mechanistic elucidation. Here we present cryogenic electron microscopy structures of human Ostα/β in apo and substrate-bound states at 2.6–3.1 Å resolution, revealing a distinctive membrane protein architecture that defines a new transporter class. Ostα/β forms a symmetric tetramer of heterodimers, with each Ostα subunit showing a new seven-transmembrane fold, augmented by a single transmembrane helix of Ostβ. This architecture is stabilized by extensive lipid modifications, including a palmitoylated cysteine-rich motif that forms a lateral substrate-binding groove. The structures uncover a unique transport pathway featuring two substrate-binding sites connected by an amphipathic helix-gated conduit. This design, conserved in the evolutionarily related TMEM184 family, suggests an ancient mechanism for substrate translocation. Electrophysiological studies demonstrate voltage-sensitive, bidirectional transport driven by electrochemical gradients, elucidating the efflux role of Ostα/β in vivo. Lipid interactions, notably palmitoylation-dependent trafficking, emerge as critical for stability and function. These findings clarify the molecular mechanism of Ostα/β, provide a structural basis for disease-associated mutations4,5 and establish a paradigm for lipid-modified membrane transport.


