2026-02-13 名古屋大学
<関連情報>
- https://www.nagoya-u.ac.jp/researchinfo/result/2026/02/post-943.html
- https://www.nagoya-u.ac.jp/researchinfo/result/upload_images/20260213_igcore.pdf
- https://www.jbc.org/article/S0021-9258(26)00083-9/fulltext
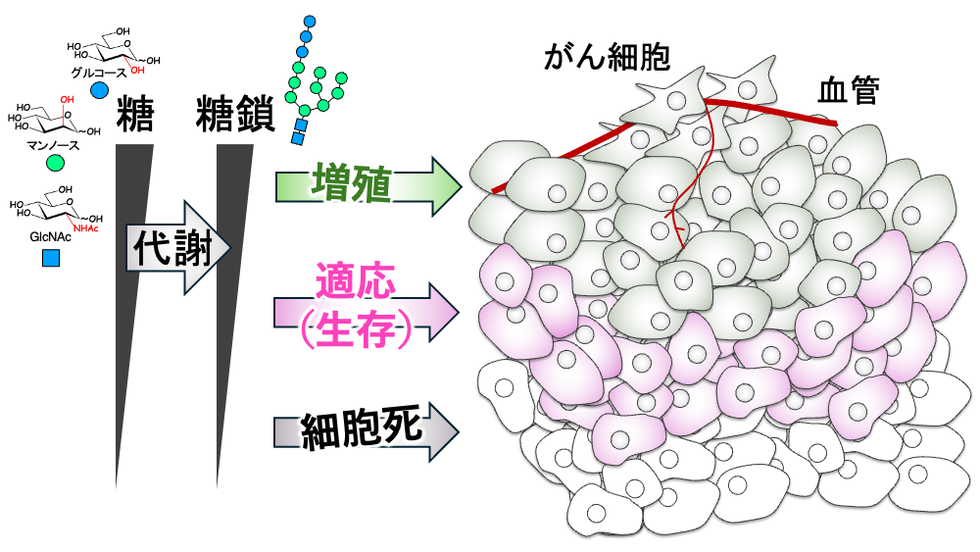
マンノース代謝経路はグルコース供給を感知し、細胞運命決定を制御する Mannose metabolic pathway senses glucose supply and regulates cell fate decisions
Ziwei Wang ∙ Yasuhide Miyamoto ∙ Takehiro Suzuki ∙ … ∙ Hideaki Tahara, ∙ Naoyuki Taniguchi ∙ Yoichiro Harada
Journal of Biological Chemistry Published:January 28, 2026
DOI:https://doi.org/10.1016/j.jbc.2026.111213
Abstract
Mammalian cells exploit diverse metabolic pathways to regulate cell fates during glucose deprivation. We previously reported that glucose deprivation lowers the metabolic activity of mannose pathway that is interconnected with glycolysis, leading to biosynthetic arrest and degradation of the glycan precursors for asparagine-linked glycosylation (N-glycosylation) in the endoplasmic reticulum (ER). However, the cellular role of this sequential metabolic response remains unknown, largely due to metabolic complications caused by glucose deprivation. Here, we genetically engineered cells to separate mannose pathway from glycolysis, allowing precise control of mannose pathway activity by adjusting mannose supply levels instead of changing glucose supply. Moderate decrease in mannose supply severely suppressed N-glycosylation, leading to activation of pro-survival PERK-eIF2 signals. Although further decrease in mannose supply to the minimal levels did not compromise cell survival, it depleted luminal protective glycocalyx of lysosomes and increased a risk of cell death by impairing lysosome integrity. These results indicate that low metabolic flux of glucose into mannose pathway initiates alterations in homeostasis of the ER and lysosomes, at least in part through N-glycosylation defects, leading to cell fate decisions.


