NF1発癌性素因症候群の腫瘍成長を促進する高活性神経細胞 Hyperactive neurons drive tumor growth in NF1 cancer predisposition syndrome
2022-05-19 ワシントン大学セントルイス
セントルイスのワシントン大学医学部の研究者らは、Nf1遺伝子に変異を有する神経細胞は過剰な興奮状態にあり、てんかんの治療薬として食品医薬品局から承認されているラモトリギンによってこの過剰な興奮状態を抑制すると、マウスにおける腫瘍の成長が止まることを発見した。
<関連情報>
- https://medicine.wustl.edu/news/epilepsy-drug-stops-nervous-system-tumor-growth-in-mice/?_ga=2.209255186.1617183991.1653618290-531768696.1642754088
- https://www.nature.com/articles/s41467-022-30466-6
神経線維腫症1型モデルにおいて、神経細胞の過剰興奮が中枢および末梢神経系腫瘍の進行を促進すること Neuronal hyperexcitability drives central and peripheral nervous system tumor progression in models of neurofibromatosis-1
Corina Anastasaki,Juan Mo,Ji-Kang Chen,Jit Chatterjee,Yuan Pan,Suzanne M. Scheaffer,Olivia Cobb,Michelle Monje,Lu Q. Le & David H. Gutmann
Nature Communications Published:19 May 2022
DOI:https://doi.org/10.1038/s41467-022-30466-6
Abstract
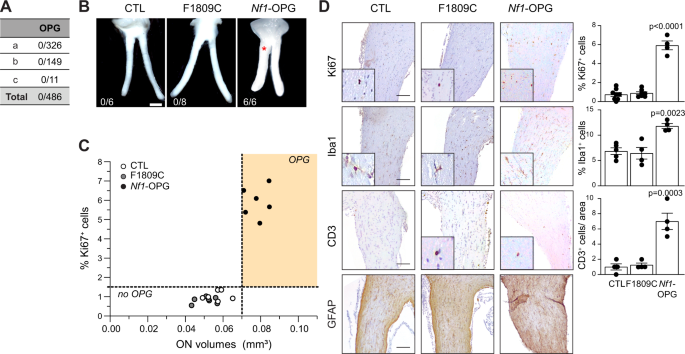
Neuronal activity is emerging as a driver of central and peripheral nervous system cancers. Here, we examined neuronal physiology in mouse models of the tumor predisposition syndrome Neurofibromatosis-1 (NF1), with different propensities to develop nervous system cancers. We show that central and peripheral nervous system neurons from mice with tumor-causing Nf1 gene mutations exhibit hyperexcitability and increased secretion of activity-dependent tumor-promoting paracrine factors. We discovered a neurofibroma mitogen (COL1A2) produced by peripheral neurons in an activity-regulated manner, which increases NF1-deficient Schwann cell proliferation, establishing that neurofibromas are regulated by neuronal activity. In contrast, mice with the Arg1809Cys Nf1 mutation, found in NF1 patients lacking neurofibromas or optic gliomas, do not exhibit neuronal hyperexcitability or develop these NF1-associated tumors. The hyperexcitability of tumor-prone Nf1-mutant neurons results from reduced NF1-regulated hyperpolarization-activated cyclic nucleotide-gated (HCN) channel function, such that neuronal excitability, activity-regulated paracrine factor production, and tumor progression are attenuated by HCN channel activation. Collectively, these findings reveal that NF1 mutations act at the level of neurons to modify tumor predisposition by increasing neuronal excitability and activity-regulated paracrine factor production.