シアノバクテリアと呼ばれる微生物の新発見は、植物科学、生物工学、環境保護に可能性をもたらす New findings in microbes called cyanobacteria present opportunities for plant science, bioengineering, and environmental protection
2022-09-01 ローレンスバークレー国立研究所(LBNL)
この研究成果は、『Nature』誌に掲載され、微生物の光合成に新たな光を当てました。特に、光エネルギーをどのように取り込み、必要な場所に送って、二酸化炭素を糖に変換するのかについてである。
今回、国際的な専門家チームと低温電子顕微鏡(クライオ電子顕微鏡)の進歩により、シアノバクテリアの集光型アンテナの構造をほぼ原子レベルの分解能で捉えることができた。
<関連情報>
- https://newscenter.lbl.gov/2022/09/01/research-team-reveals-a-blueprint-for-photosynthesis/
- https://www.nature.com/articles/s41586-022-05156-4
光捕集状態および光保護状態のフィコビリソームの構造 Structures of a phycobilisome in light-harvesting and photoprotected states
María Agustina Domínguez-Martín,Paul V. Sauer,Henning Kirst,Markus Sutter,David Bína,Basil J. Greber,Eva Nogales,Tomáš Polívka & Cheryl A. Kerfeld
Nature Published:31 August 2022
DOI:https://doi.org/10.1038/s41586-022-05156-4
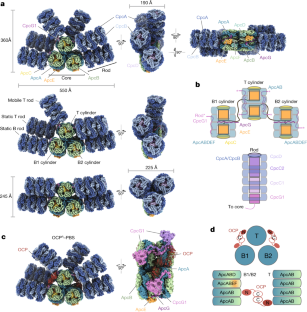
Abstract
Phycobilisome (PBS) structures are elaborate antennae in cyanobacteria and red algae1,2. These large protein complexes capture incident sunlight and transfer the energy through a network of embedded pigment molecules called bilins to the photosynthetic reaction centres. However, light harvesting must also be balanced against the risks of photodamage. A known mode of photoprotection is mediated by orange carotenoid protein (OCP), which binds to PBS when light intensities are high to mediate photoprotective, non-photochemical quenching3,4,5,6. Here we use cryogenic electron microscopy to solve four structures of the 6.2 MDa PBS, with and without OCP bound, from the model cyanobacterium Synechocystis sp. PCC 6803. The structures contain a previously undescribed linker protein that binds to the membrane-facing side of PBS. For the unquenched PBS, the structures also reveal three different conformational states of the antenna, two previously unknown. The conformational states result from positional switching of two of the rods and may constitute a new mode of regulation of light harvesting. Only one of the three PBS conformations can bind to OCP, which suggests that not every PBS is equally susceptible to non-photochemical quenching. In the OCP–PBS complex, quenching is achieved through the binding of four 34 kDa OCPs organized as two dimers. The complex reveals the structure of the active form of OCP, in which an approximately 60 Å displacement of its regulatory carboxy terminal domain occurs. Finally, by combining our structure with spectroscopic properties7, we elucidate energy transfer pathways within PBS in both the quenched and light-harvesting states. Collectively, our results provide detailed insights into the biophysical underpinnings of the control of cyanobacterial light harvesting. The data also have implications for bioengineering PBS regulation in natural and artificial light-harvesting systems.


