炎症に関する新たな研究により、進行がんやこれまで治療できなかったがんの患者さんの予後を改善するためのより良い治療法につながる可能性があります。 New research on inflammation could lead to better treatments to improve outcomes for people with advanced or previously untreatable cancers.
2023-03-14 ニューサウスウェールズ大学(UNSW)
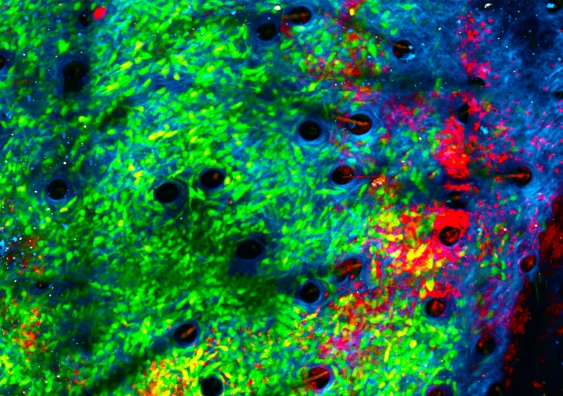
When exposed to bacteria, neutrophils (red) began attacking cancer cells (green) in the collagen structure (blue) of a tumour’s microenvironment. Image credit: Jacqueline Bailey, Dr Chtanova’s Innate Tumour Immunology Lab, Garvan
シドニー校とGarvan医療研究所の研究者たちは、腫瘍の微小環境に細菌を導入することで急性炎症状態を作り出し、免疫系の最初の対応細胞が腫瘍を攻撃するようになることを発見しました。
白血球の一種である好中球は、感染防御に重要な役割を果たしますが、腫瘍成長を促進することでも有名です。腫瘍周辺の微小環境にStaphylococcus aureus微生物の不活性サンプルを注入すると、好中球の保護機能が反転し、腫瘍を攻撃するようになります。
この研究は、Cancer Research誌に掲載されました。研究により、急性炎症が抗腫瘍機能を持つ免疫細胞を継続的に実現するために利用できることが示され、微生物療法がチェックポイント阻害剤療法と組み合わせることで抗がん能力を増幅することができることが分かりました。研究者たちは、現在、転移したがんを治療する方法を開発するために、これらの発見を拡張しようとしています。
<関連情報>
- https://newsroom.unsw.edu.au/news/science-tech/exposing-tumours-bacteria-converts-immune-cells-cancer-killers
- https://aacrjournals.org/cancerres/article/doi/10.1158/0008-5472.CAN-21-4025/716558/Neutrophil-conversion-to-a-tumor-killing-phenotype
好中球の殺腫瘍性表現型への転換が効果的な微生物療法を支える Neutrophil conversion to a tumor-killing phenotype underpins effective microbial therapy
Andrew O. Yam,Jacqueline Bailey,Francis Lin,Arnolda Jakovija,Scott E. Youlten,Claudio Counoupas,Matthias Gunzer,Tobias Bald,Trent M. Woodruff,James A. Triccas,Leonard D. Goldstein,David Gallego-Ortega,Shane T. Grey,Tatyana Chtanova
Cancer Research Published:February 14 2023
DOI:https://doi.org/10.1158/0008-5472.CAN-21-4025
Abstract
The inflammatory microenvironment of solid tumors creates a pro-tumorigenic milieu that resembles chronic inflammation akin to a subverted wound healing response. Here we investigated the effect of converting the tumor microenvironment from a chronically inflamed state to one of acute microbial inflammation by injecting microbial bioparticles directly into tumors. Intratumoral microbial bioparticle injection led to rapid and dramatic changes in the tumor immune composition, the most striking of which was a substantial increase in the presence of activated neutrophils. In situ photoconversion and intravital microscopy indicated that tumor neutrophils transiently switched from sessile producers of vascular endothelial growth factor to highly motile neutrophils that clustered to make neutrophil-rich domains in the tumor. The neutrophil clusters remodeled tumor tissue and repressed tumor growth. Single cell transcriptional analysis of microbe-stimulated neutrophils showed a profound shift in gene expression towards heightened activation and anti-microbial effector function. Microbe-activated neutrophils also upregulated chemokines known to regulate neutrophil and CD8+ T cell recruitment. Microbial therapy also boosted CD8+ T cell function and enhanced the therapeutic benefit of checkpoint inhibitor therapy in tumor-bearing mice and provided protection in a model of tumor recurrence. These data indicate that one of the major effector mechanisms of microbial therapy is the conversion of tumor neutrophils from a wound healing to an acutely activated cytotoxic phenotype, highlighting a rationale for broader deployment of microbial therapy in the treatment of solid cancers.


