2023-06-12 ヒューストン大学(UH)
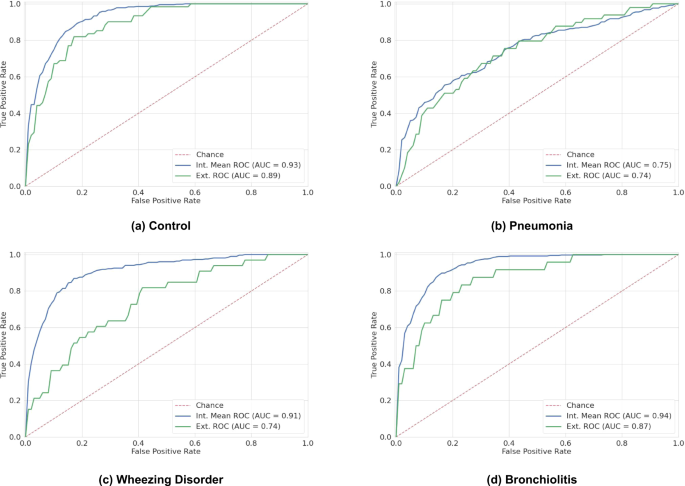
◆アンドロゲン欠乏療法に反応しなくなったCRPC患者の細胞で、特定の化学修飾と関連するTRAF4タンパク質の増加が観察されました。これらの結果は、ARの修飾によって引き起こされる特定の分子変化を標的とした治療が、一部のCRPC患者に効果的な治療法となり得る可能性を示しています。
<関連情報>
- https://uh.edu/news-events/stories/2023/june-2023/06122023-prostate-cancer-hormone-resistant.php
- https://www.pnas.org/doi/10.1073/pnas.2218229120
TRAF4を介したアンドロゲン受容体の非保護分解的ユビキチン化は去勢抵抗性前立腺がんを促進する TRAF4-mediated nonproteolytic ubiquitination of androgen receptor promotes castration-resistant prostate cancer
Ramesh Singh , Huan Meng , Tao Shen, Lance Edward V. Lumahan , Steven Nguyen , Hong Shen, Subhamoy Dasgupta , Li Qin , Dileep Karri, Bokai Zhu , Feng Yang , Cristian Coarfa, Bert W. O’Malley , and Ping Yi
Proceedings of the National Academy of Sciences Published:May 8, 2023
DOI:https://doi.org/10.1073/pnas.2218229120
Significance
We identified an AR ubiquitination site undergoing TRAF4-mediated nonclassical K27-linked ubiquitination under castration conditions. This ubiquitination enhances the interaction between AR and the pioneer factor FOXA1 and triggers a switch of AR genomic binding and AR-regulated transcriptome. Ubiquitinated AR subsequently hyperactivates E2F to promote CRPC. Our studies lay the foundation for identifying an appropriate CRPC patient population sensitive to CDK4/6 inhibitor treatment.
Abstract
Castration-resistant prostate cancer (CRPC) poses a major clinical challenge with the androgen receptor (AR) remaining to be a critical oncogenic player. Several lines of evidence indicate that AR induces a distinct transcriptional program after androgen deprivation in CRPCs. However, the mechanism triggering AR binding to a distinct set of genomic loci in CRPC and how it promotes CRPC development remain unclear. We demonstrate here that atypical ubiquitination of AR mediated by an E3 ubiquitin ligase TRAF4 plays an important role in this process. TRAF4 is highly expressed in CRPCs and promotes CRPC development. It mediates K27-linked ubiquitination at the C-terminal tail of AR and increases its association with the pioneer factor FOXA1. Consequently, AR binds to a distinct set of genomic loci enriched with FOXA1- and HOXB13-binding motifs to drive different transcriptional programs including an olfactory transduction pathway. Through the surprising upregulation of olfactory receptor gene transcription, TRAF4 increases intracellular cAMP levels and boosts E2F transcription factor activity to promote cell proliferation under androgen deprivation conditions. Altogether, these findings reveal a posttranslational mechanism driving AR-regulated transcriptional reprogramming to provide survival advantages for prostate cancer cells under castration conditions.