2023-06-15 カリフォルニア大学サンディエゴ校(UCSD)
◆この発見は、がんの治療法の開発に役立つ可能性があります。さらに、破壊されたDNA断片のつながりを阻止することで、がん細胞の形成を減らすことができるかもしれません。この研究は、クロモソームの安定性に影響を与えるタンパク質を治療の標的とすることが提案されています。
<関連情報>
- https://today.ucsd.edu/story/tethering-of-shattered-chromosomal-fragments-paves-way-for-new-cancer-therapies
- https://www.nature.com/articles/s41586-023-06216-z#Ack1
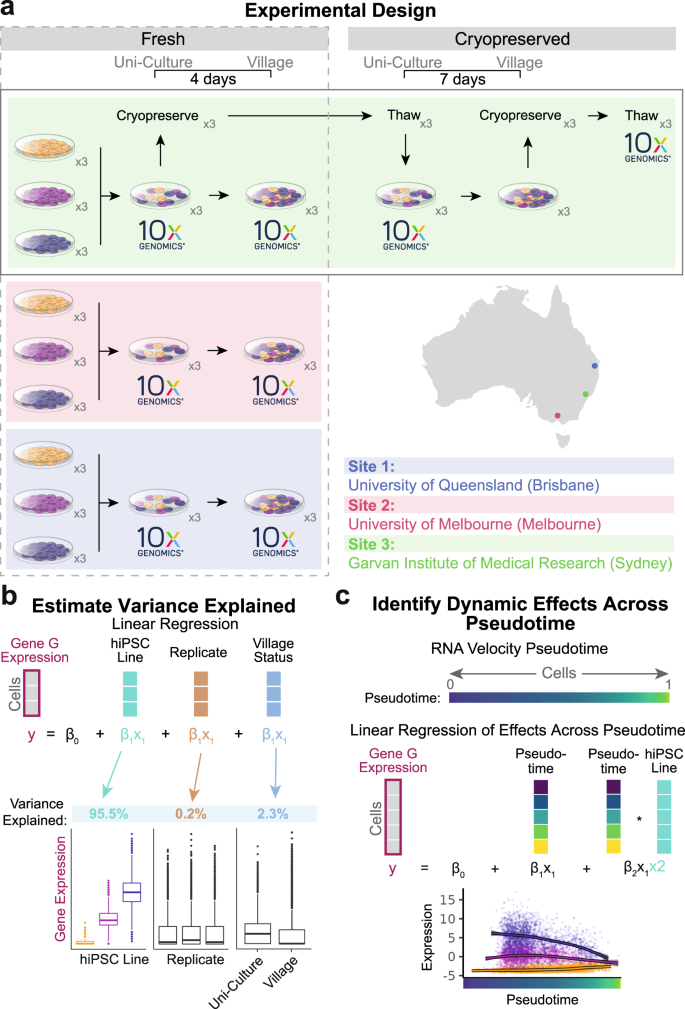
分裂期のテザーが砕けた微小核染色体の継承を可能にする Mitotic tethering enables inheritance of shattered micronuclear chromosomes
Prasad Trivedi,Christopher D. Steele,Franco K. C. Au,Ludmil B. Alexandrov & Don W. Cleveland
Nature Published:14 June 2023
DOI:https://doi.org/10.1038/s41586-023-06216-z
Abstract
Chromothripsis, the shattering and imperfect reassembly of one (or a few) chromosome(s)1, is an ubiquitous2 mutational process generating localized and complex chromosomal rearrangements that drive genome evolution in cancer. Chromothripsis can be initiated by mis-segregation errors in mitosis3,4 or DNA metabolism5,6,7 that lead to entrapment of chromosomes within micronuclei and their subsequent fragmentation in the next interphase or following mitotic entry6,8,9,10. Here we use inducible degrons to demonstrate that chromothriptically produced pieces of a micronucleated chromosome are tethered together in mitosis by a protein complex consisting of mediator of DNA damage checkpoint 1 (MDC1), DNA topoisomerase II-binding protein 1 (TOPBP1) and cellular inhibitor of PP2A (CIP2A), thereby enabling en masse segregation to the same daughter cell. Such tethering is shown to be crucial for the viability of cells undergoing chromosome mis-segregation and shattering after transient inactivation of the spindle assembly checkpoint. Transient, degron-induced reduction in CIP2A following chromosome micronucleation-dependent chromosome shattering is shown to drive acquisition of segmental deletions and inversions. Analyses of pancancer tumour genomes showed that expression of CIP2A and TOPBP1 was increased overall in cancers with genomic rearrangements, including copy number-neutral chromothripsis with minimal deletions, but comparatively reduced in cancers with canonical chromothripsis in which deletions were frequent. Thus, chromatin-bound tethers maintain the proximity of fragments of a shattered chromosome enabling their re-encapsulation into, and religation within, a daughter cell nucleus to form heritable, chromothriptically rearranged chromosomes found in the majority of human cancers.