2023-08-11 ペンシルベニア州立大学(PennState)
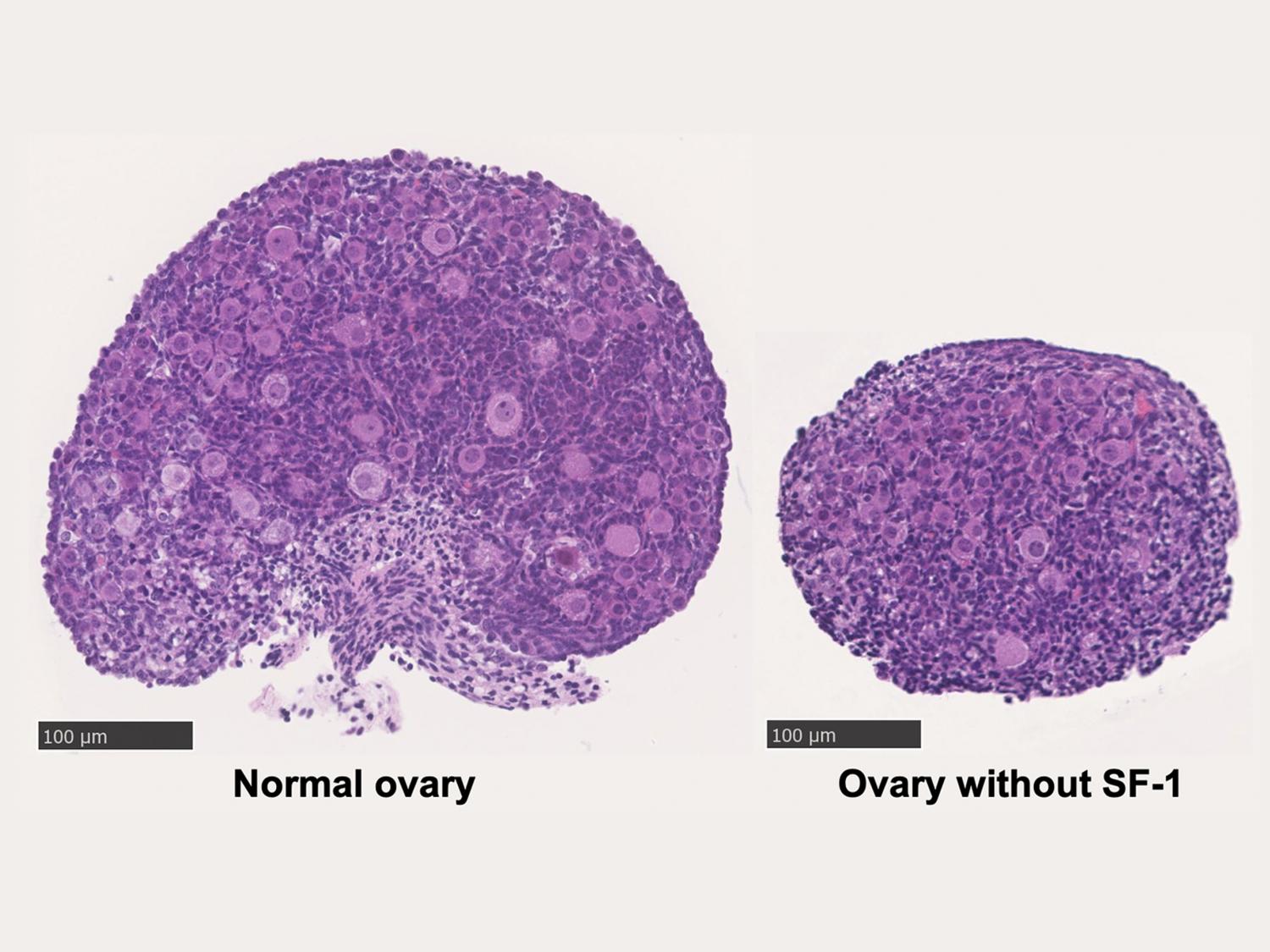 Without the SF-1 protein, ovaries do not grow and have far fewer eggs. On the left is a normal mouse ovary from a four-day-old mouse and on the right is an ovary without SF-1, also from a from a four-day-old mouse. Credit: Camilla Hughes. All Rights Reserved.
Without the SF-1 protein, ovaries do not grow and have far fewer eggs. On the left is a normal mouse ovary from a four-day-old mouse and on the right is an ovary without SF-1, also from a from a four-day-old mouse. Credit: Camilla Hughes. All Rights Reserved.
◆この発見は、女性の早発性卵巣不全や更年期、乳牛の生殖問題の研究基盤となる。SF-1の欠如で卵巣卵胞数が減少し、卵胞の形成が障害されることが示された。この成果は、人間と動物の生殖健康の理解と治療開発に影響を与える可能性がある。
<関連情報>
- https://www.psu.edu/news/research/story/newly-identified-protein-function-may-reveal-understanding-lifetime-fertility/
- https://www.pnas.org/doi/abs/10.1073/pnas.2220849120
ステロイド形成因子1(SF-1; Nr5a1)は卵巣予備能の形成を制御する Steroidogenic factor 1 (SF-1; Nr5a1) regulates the formation of the ovarian reserve
Camilla H. K. Hughes,Olivia E. Smith,Marie-Charlotte Meinsohn,Mylène Brunelle,Nicolas Gévry,and Bruce D. Murphy
Proceedings of the National Academy of Sciences Published:July 26, 2023
DOI:https://doi.org/10.1073/pnas.2220849120
Significance
In this study, we identified the orphan nuclear receptor Steroidogenic factor 1 (SF-1) as a regulator of the formation of the ovarian follicle reserve, which is composed of all the primordial oocytes and the somatic cells that surround and support them. Depletion of SF-1 resulted in dramatically reduced ovarian reserve due to impaired follicular formation and increased oocyte death. This was caused by dysregulated deposition of ovarian laminins, impaired oocyte–granulosa cell communication, and disrupted KIT-KITL and Notch signaling. This study reveals an important mechanism of ovarian reserve establishment, indicating that SF-1 is a key factor in establishing the reserve of follicles that determines lifetime fertility. Therefore, this work provides a foundation for future investigations of premature ovarian insufficiency and menopause.
Abstract
The ovarian follicle reserve, formed pre- or perinatally, comprises all oocytes for lifetime reproduction. Depletion of this reserve results in infertility. Steroidogenic factor 1 (SF-1; Nr5a1) and liver receptor homolog 1 (LRH-1; Nr5a2) are two orphan nuclear receptors that regulate adult endocrine function, but their role in follicle formation is unknown. We developed models of conditional depletion of SF-1 or LRH-1 from prenatal ovaries. Depletion of SF-1, but not LRH-1, resulted in dramatically smaller ovaries and fewer primordial follicles. This was mediated by increased oocyte death, resulting from increased ovarian inflammation and increased Notch signaling. Major dysregulated genes were Iroquois homeobox 3 and 5 and their downstream targets involved in the establishment of the ovarian laminin matrix and oocyte-granulosa cell gap junctions. Disruptions of these pathways resulted in follicles with impaired basement membrane formation and compromised oocyte–granulosa communication networks, believed to render them more prone to atresia. This study identifies SF-1 as a key regulator of the formation of the ovarian reserve.

