2023-08-25 韓国基礎科学研究院(IBS)
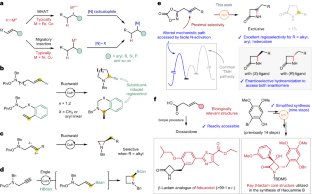
◆彼らは、低コストのニッケル触媒を使用して、高い選択的性能でキラルなβ-ラクタムを合成する方法を開発し、これにより複雑な化合物の合成が容易になりました。この新しい方法は、薬物や天然物質の合成を効率化し、新しい薬物候補の合成も可能になりました。この成果は、薬物開発を大幅に短縮する可能性を持つ革新的なものであり、低コストで高付加価値の物質を合成するための新たな道を開拓しました。
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do
- https://www.nature.com/articles/s41929-023-01014-2
アルケンの分子内ヒドロアミド化により、転置型NiH触媒を用いたβ-ラクタムの不斉合成が可能になる Intramolecular hydroamidation of alkenes enabling asymmetric synthesis of β-lactams via transposed NiH catalysis
Xiang Lyu,Changhyeon Seo,Hoimin Jung,Teresa Faber,Dongwook Kim,Sangwon Seo & Sukbok Chang
Nature Catalysis Published:24 August 2023
DOI:https://doi.org/10.1038/s41929-023-01014-2
Abstract
Synthetic methods for constructing enantioenriched β-lactams are highly valuable given their ubiquity in bioactive compounds, most notably in antibiotics such as penicillins and carbapenems. Intramolecular hydroamidation of β,γ-unsaturated amides would provide a convenient means to reach this alluring chemical space, yet it remains limited due to the regioselectivity issue arising from the difficulty associated with the formation of strained four-membered rings. Here we describe a NiH-catalysed strategy that addresses this challenge through the use of readily accessible alkenyl dioxazolone derivatives. The reaction transcends the conventional NiH operation mode via a transposed mechanism initiated by N-activation, thus allowing for proximal C–N bond formation with excellent regioselectivity, regardless of the electronic properties of substituents. This mechanistic platform is also highly effective for the enantioselective intramolecular hydroamidation of alkenes to enable a convenient access to enantioenriched β-lactams.