2023-10-09 ミシガン大学
◆ミシガン大学で開発されたこの技術は、肝臓組織を破壊するために超音波を使用し、臨床試験では効果と安全性が証明されています。この技術は、がん治療において薬物療法や放射線療法の補完として使用され、患者にとって負担の少ない治療方法を提供する可能性があります。また、ヒストトリプシーは免疫系にがん細胞を識別させる効果を持っており、将来的にがん治療の進化に寄与する可能性があります。
<関連情報>
- https://news.umich.edu/tumor-destroying-sound-waves-receive-fda-approval-for-liver-treatment-in-humans/
- https://www.frontiersin.org/articles/10.3389/fimmu.2023.1012799/full
- https://www.mdpi.com/2072-6694/14/7/1612
ヒストトリプシー集束超音波腫瘍治療に対する時空間的な局所的・終局的な細胞死と免疫反応 Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation
Ashley L. Pepple,Joey L. Guy, Reliza McGinnis, Amy E. Felsted,Brian Song,Ryan Hubbard,Tejaswi Worlikar,Hannah Garavaglia,Joe Dib,Hannah Chao,Nicoleen Boyle,Michal Olszewski,Zhen Xu,Anutosh Ganguly,Clifford S. Cho
Frontiers in Immunology Published:23 January 2023
DOI:https://doi.org/10.3389/fimmu.2023.1012799
Introduction: Histotripsy is a novel focused ultrasound tumor ablation modality with potent immunostimulatory effects.
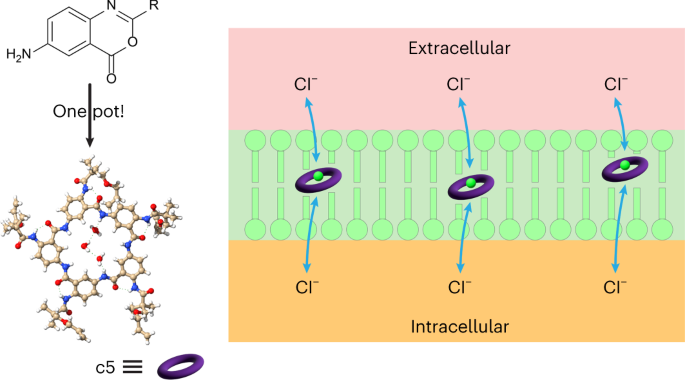
Methods: To measure the spatiotemporal kinetics of local andabscopal responses to histotripsy, C57BL/6 mice bearing bilateral flank B16 melanoma or Hepa1-6 hepatocellular carcinoma tumors were treated with unilateral sham or partial histotripsy. Treated and contralateral untreated (abscopal) tumors were analyzed using multicolor immunofluorescence, digital spatial profiling, RNA sequencing (RNASeq), and flow cytometry.
Results: Unilateral histotripsy triggered abscopal tumor growth inhibition. Within the ablation zone, early high mobility group box protein 1 (HMGB1) release and necroptosis were accompanied by immunogenic cell death transcriptional responses in tumor cells and innate immune activation transcriptional responses in infiltrating myeloid and natural killer (NK) cells. Delayed CD8+ T cell intratumoral infiltration was spatiotemporally aligned with cancer cell features of ferroptosis; this effect was enhanced by CTLA-4 blockade and recapitulated in vitro when tumor-draining lymph node CD8+ T cells were co-cultured with tumor cells. Inoculation with cell-free tumor fractions generated by histotripsy but not radiation or freeze/thaw conferred partial protection from tumor challenge.
Discussion: We propose that histotripsy may evoke local necroptotic immunogenic cell death, priming systemic adaptive immune responses and abscopal ferroptotic cancer cell death.
齧歯類肝腫瘍モデルにおける肝内転移の発生に対するヒストトリプシーの影響 Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model
Tejaswi Worlikar,,Man Zhang,,Anutosh Ganguly,,Timothy L. Hall,,Jiaqi Shi,,Lili Zhao,,Fred T. Lee,,Mishal Mendiratta-Lala,,Clifford S. Cho and Zhen Xu
Cancers Published: 22 March 2022
DOI:https://doi.org/10.3390/cancers14071612
Simple Summary
Histotripsy is a novel technique that mechanically disrupts tumors, through precisely controlled acoustic cavitation. There is insufficient evidence regarding the effects of histotripsy on the risk of recurrence and metastases, following tumor debulking. The aim of this study is to evaluate the effect of partial histotripsy tumor ablation (~50–75% tumor volume targeted) on untargeted tumor progression, survival outcomes, risk of metastases and immune infiltration, in an orthotopic, immunocompetent, metastatic rodent hepatocellular carcinoma (HCC) model. Even with partial ablation, complete local tumor regression was observed in 81% of treatment rats, with no recurrence or metastasis. In contrast, 100% of the untreated control animals showed local tumor progression and intrahepatic metastases. Histotripsy-treated animals had statistically significant improved survival outcomes compared to controls (p-value < 0.0001). Histotripsy-treated animals had increased immune infiltration, as compared to controls, which may have contributed to the eventual regression of the untargeted tumor region in partial histotripsy-treated tumors.
Abstract
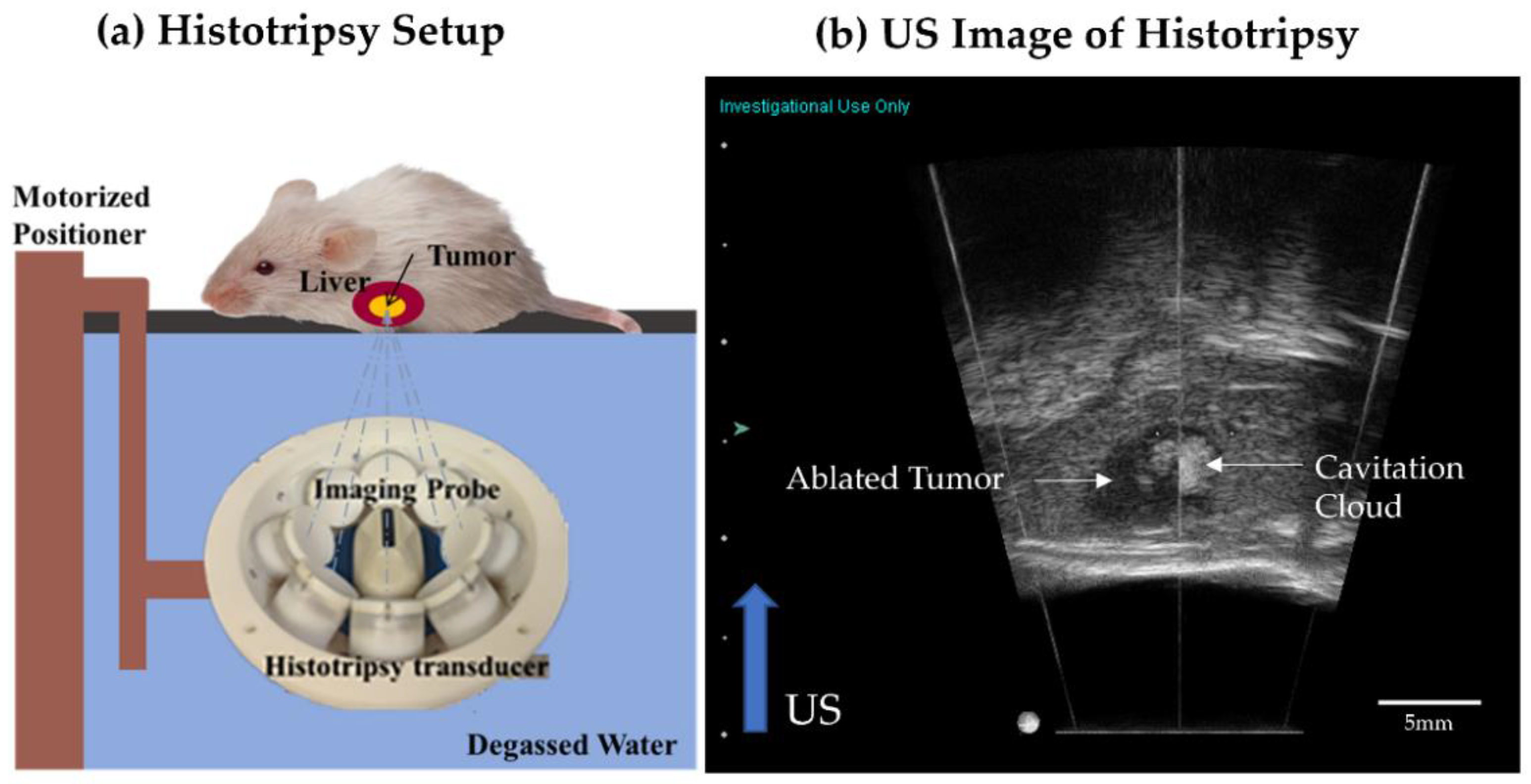
Histotripsy has been used for tumor ablation, through controlled, non-invasive acoustic cavitation. This is the first study to evaluate the impact of partial histotripsy ablation on immune infiltration, survival outcomes, and metastasis development, in an in vivo orthotopic, immunocompetent rat HCC model (McA-RH7777). At 7–9 days post-tumor inoculation, the tumor grew to 5–10 mm, and ~50–75% tumor volume was treated by ultrasound-guided histotripsy, by delivering 1–2 cycle histotripsy pulses at 100 Hz PRF (focal peak negative pressure P– >30 MPa), using a custom 1 MHz transducer. Complete local tumor regression was observed on MRI in 9/11 histotripsy-treated rats, with no local recurrence or metastasis up to the 12-week study end point, and only a <1 mm residual scar tissue observed on histology. In comparison, 100% of untreated control animals demonstrated local tumor progression, developed intrahepatic metastases, and were euthanized at 1–3 weeks. Survival outcomes in histotripsy-treated animals were significantly improved compared to controls (p-value < 0.0001). There was evidence of potentially epithelial-to-mesenchymal transition (EMT) in control tumor and tissue healing in histotripsy-treated tumors. At 2- and 7-days post-histotripsy, increased immune infiltration of CD11b+, CD8+ and NK cells was observed, as compared to controls, which may have contributed to the eventual regression of the untargeted tumor region in histotripsy-treated tumors.