2024-01-22 ミュンヘン大学(LMU)
◆樹状細胞が生成するCCL17がT細胞をどのように調節するかについての新たなメカニズムが明らかにされ、慢性炎症性疾患の治療における重要な手がかりとなる可能性が示唆されています。特に、この研究は治療介入の出発点となり、心血管疾患や炎症性疾患の治療法の向上に寄与する可能性があります。
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/new-signaling-pathway-uncovered-shedding-fresh-light-on-atherosclerosis.html
- https://www.nature.com/articles/s44161-023-00413-9
動脈硬化を促進する制御性T細胞を抑制する非正規ケモカイン受容体経路の同定 Identification of a non-canonical chemokine-receptor pathway suppressing regulatory T cells to drive atherosclerosis
Yvonne Döring,Emiel P. C. van der Vorst,Yi Yan,Carlos Neideck,Xavier Blanchet,Yvonne Jansen,Manuela Kemmerich,Soyolmaa Bayasgalan,Linsey J. F. Peters,Michael Hristov,Kiril Bidzhekov,Changjun Yin,Xi Zhang,Julian Leberzammer,Ya Li,Inhye Park,Maria Kral,Katrin Nitz,Laura Parma,Selin Gencer,Andreas J. R. Habenicht,Alexander Faussner,Daniel Teupser,Claudia Monaco,… Christian Weber
Nature Cardiovascular Research Published:22 January 2024
DOI:https://doi.org/10.1038/s44161-023-00413-9
Abstract
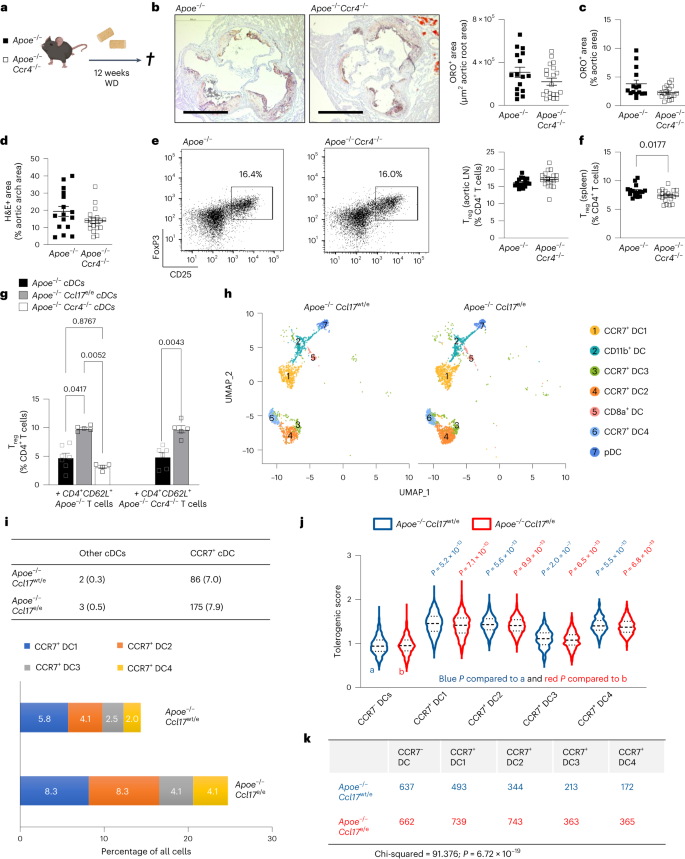
CCL17 is produced by conventional dendritic cells, signals through CCR4 on regulatory T (Treg) cells and drives atherosclerosis by suppressing Treg functions through yet undefined mechanisms. Here we show that conventional dendritic cells from CCL17-deficient mice display a pro-tolerogenic phenotype and transcriptome that is not phenocopied in mice lacking its cognate receptor CCR4. In the plasma of CCL17-deficient mice, CCL3 was the only decreased cytokine/chemokine. We found that CCL17 signaled through CCR8 as an alternate high-affinity receptor, which induced CCL3 expression and suppressed Treg functions in the absence of CCR4. Genetic ablation of CCL3 and CCR8 in CD4+ T cells reduced CCL3 secretion, boosted FoxP3+ Treg numbers and limited atherosclerosis. Conversely, CCL3 administration exacerbated atherosclerosis and restrained Treg differentiation. In symptomatic versus asymptomatic human carotid atheroma, CCL3 expression was increased, whereas FoxP3 expression was reduced. Together, we identified a non-canonical chemokine pathway whereby CCL17 interacts with CCR8 to yield a CCL3-dependent suppression of atheroprotective Treg cells.