2024-05-07 ペンシルベニア州立大学(PennState)
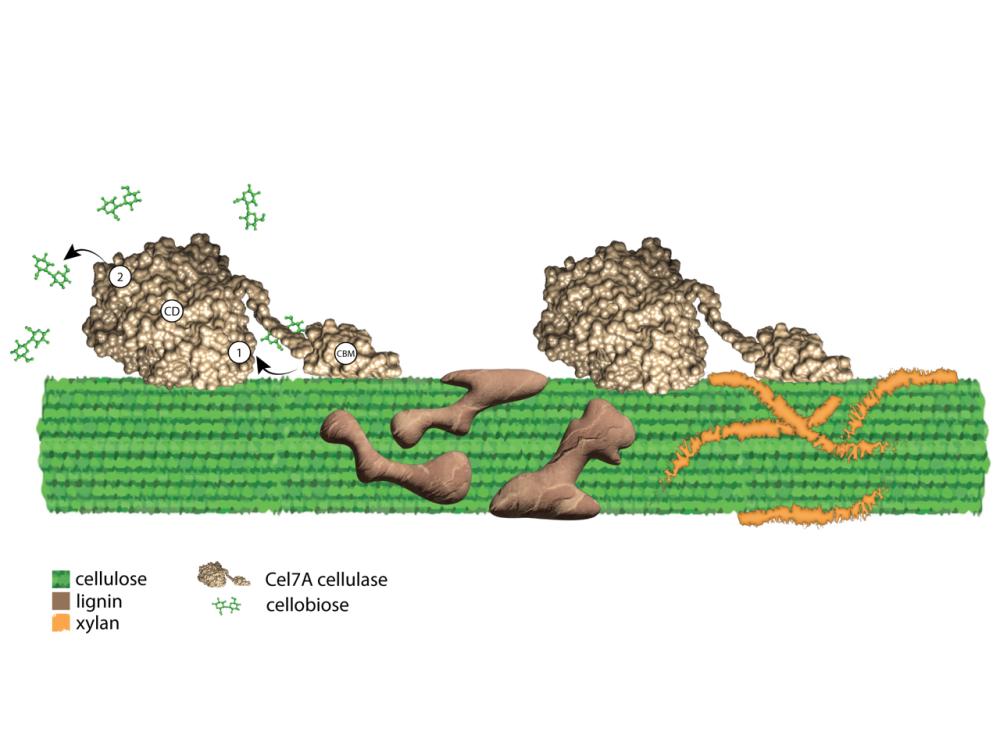 The research team identified new details about how Cel7A cellulase enzymes (gold) are inhibited when breaking down cellulose (green) by the product of cellulose degradation, cellobiose, at the “front door” (1) and “back door” (2) of the Cel7A catalytic tunnel, and by two other components of plant cell walls, lignin (brown) and xylan (orange), that interact with cellulose. This research promises to reveal new strategies for efficiently deconstructing cellulose to produce sustainable bioenergy and biomaterials. Credit: Nerya Zexer / Penn State. Creative Commons
The research team identified new details about how Cel7A cellulase enzymes (gold) are inhibited when breaking down cellulose (green) by the product of cellulose degradation, cellobiose, at the “front door” (1) and “back door” (2) of the Cel7A catalytic tunnel, and by two other components of plant cell walls, lignin (brown) and xylan (orange), that interact with cellulose. This research promises to reveal new strategies for efficiently deconstructing cellulose to produce sustainable bioenergy and biomaterials. Credit: Nerya Zexer / Penn State. Creative Commons
<関連情報>
- https://www.psu.edu/news/eberly-college-science/story/why-breaking-down-plant-material-biofuels-so-slow/
- https://www.pnas.org/doi/10.1073/pnas.2322567121
単一分子追跡により、Cel7Aセルラーゼの生成物セロビオースによるフロントドア/バックドア二重阻害が明らかになった Single-molecule tracking reveals dual front door/back door inhibition of Cel7A cellulase by its product cellobiose
Daguan Nong, Zachary K. Haviland, Nerya Zexer, +4, and William O. Hancock
Proceedings of the National Academy of Sciences Published:April 22, 2024
DOI:https://doi.org/10.1073/pnas.2322567121
Significance
Cellulose, a polymer of repeating glucose subunits, is the primary component of plant cell walls. A promising route to reducing petrochemical use is digesting plant biomass to glucose and fermenting glucose to bioethanol. Cel7A is a model cellulase enzyme that degrades cellulose from one end to generate the disaccharide product, cellobiose. Because industrial-scale bioethanol generation generates high concentrations of cellobiose, product inhibition is a significant concern. We investigated product inhibition of Cel7A by cellobiose at the single-molecule level and found that cellobiose both slows the movement of Cel7 along cellulose and inhibits the initial binding of Cel7 to cellulose. These results suggest that cellobiose binds to the enzyme at more than one site and achieves its inhibition by multiple mechanisms.
Abstract
Degrading cellulose is a key step in the processing of lignocellulosic biomass into bioethanol. Cellobiose, the disaccharide product of cellulose degradation, has been shown to inhibit cellulase activity, but the mechanisms underlying product inhibition are not clear. We combined single-molecule imaging and biochemical investigations with the goal of revealing the mechanism by which cellobiose inhibits the activity of Trichoderma reesei Cel7A, a well-characterized exo-cellulase. We find that cellobiose slows the processive velocity of Cel7A and shortens the distance moved per encounter; effects that can be explained by cellobiose binding to the product release site of the enzyme. Cellobiose also strongly inhibits the binding of Cel7A to immobilized cellulose, with a Ki of 2.1 mM. The isolated catalytic domain (CD) of Cel7A was also inhibited to a similar degree by cellobiose, and binding of an isolated carbohydrate-binding module to cellulose was not inhibited by cellobiose, suggesting that cellobiose acts on the CD alone. Finally, cellopentaose inhibited Cel7A binding at micromolar concentrations without affecting the enzyme’s velocity of movement along cellulose. Together, these results suggest that cellobiose inhibits Cel7A activity both by binding to the “back door” product release site to slow activity and to the “front door” substrate-binding tunnel to inhibit interaction with cellulose. These findings point to strategies for engineering cellulases to reduce product inhibition and enhance cellulose degradation, supporting the growth of a sustainable bioeconomy.