2024-05-17 ワシントン大学セントルイス校
 Researchers at Washington University School of Medicine in St. Louis have found that a strain of gut bacteria can boost immune responses and enhance cancer immunotherapy to fight sarcoma tumors in mice. Shown is a rendering of microbes in the intestine. (Image: Getty Images)
Researchers at Washington University School of Medicine in St. Louis have found that a strain of gut bacteria can boost immune responses and enhance cancer immunotherapy to fight sarcoma tumors in mice. Shown is a rendering of microbes in the intestine. (Image: Getty Images)
◆研究では、腸内細菌が免疫システムを動員し、腫瘍を攻撃する役割があることを示しました。特定の腸内細菌を使ったプロバイオティクスが、免疫療法の効果を向上させる可能性があります。次のステップとして、R. gnavusがどのように腫瘍を拒絶するかを理解し、新たな治療法の開発を目指しています。
<関連情報>
- https://source.wustl.edu/2024/05/gut-bacteria-boost-immune-response-to-fight-tumors/
- https://www.science.org/doi/10.1126/sciimmunol.adi5374
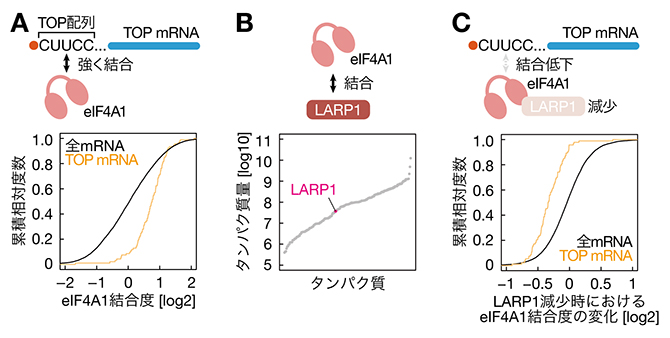
TREM2欠損は腸管マクロファージと微生物叢を再プログラムし、抗PD-1腫瘍免疫療法を強化する TREM2 deficiency reprograms intestinal macrophages and microbiota to enhance anti–PD-1 tumor immunotherapy
BLANDA DI LUCCIA, MARTINA MOLGORA, DARYA KHANTAKOVA, NATALIA JAEGER, […], AND MARCO COLONNA
Science Immunology Published:17 May 2024
DOI:https://doi.org/10.1126/sciimmunol.adi5374
Editor’s summary
The composition of the gut microbiome affects responses of patients with cancer to immune checkpoint blockade, but the microbiome’s involvement in myeloid cell–targeted immunotherapies remains unclear. Di Luccia et al. found that mice lacking the macrophage receptor TREM2 are more responsive to anti–PD-1 tumor immunotherapy because of changes that occur in their gut microbiota. TREM2-deficient mice treated with anti–PD-1 showed expansion of the Gram-positive bacterium Ruminococcus gnavus. Oral treatment of mice with R. gnavus enhanced gut-derived T cell migration to tumors, macrophage proinflammatory gene expression, and tumor responses to anti–PD-1. These findings demonstrate that expansion of specific bacterial species, such as R. gnavus in TREM2-deficient mice, can drive enhanced tumor responses to anti–PD-1 immunotherapy. —Claire Olingy
Abstract
The gut microbiota and tumor-associated macrophages (TAMs) affect tumor responses to anti–programmed cell death protein 1 (PD-1) immune checkpoint blockade. Reprogramming TAM by either blocking or deleting the macrophage receptor triggering receptor on myeloid cells 2 (TREM2) attenuates tumor growth, and lack of functional TREM2 enhances tumor elimination by anti–PD-1. Here, we found that anti–PD-1 treatment combined with TREM2 deficiency in mice induces proinflammatory programs in intestinal macrophages and a concomitant expansion of Ruminococcus gnavus in the gut microbiota. Gavage of wild-type mice with R. gnavus enhanced anti–PD-1–mediated tumor elimination, recapitulating the effect occurring in the absence of TREM2. A proinflammatory intestinal environment coincided with expansion, increased circulation, and migration of TNF-producing CD4+ T cells to the tumor bed. Thus, TREM2 remotely controls anti–PD-1 immune checkpoint blockade through modulation of the intestinal immune environment and microbiota, with R. gnavus emerging as a potential probiotic agent for increasing responsiveness to anti-PD-1.