2024-06-19 シンガポール国立大学(NUS)
<関連情報>
- https://news.nus.edu.sg/synthesis-sugar-compounds-nature-inspired-approach/
- https://www.nature.com/articles/s41586-024-07548-0
天然糖の直接ラジカル官能基化 Direct radical functionalization of native sugars
Yi Jiang,Yi Wei,Qian-Yi Zhou,Guo-Quan Sun,Xia-Ping Fu,Nikita Levin,Yijun Zhang,Wen-Qiang Liu,NingXi Song,Shabaz Mohammed,Benjamin G. Davis & Ming Joo Koh
Nature Published:19 June 2024
DOI:https://doi.org/10.1038/s41586-024-07548-0
Abstract
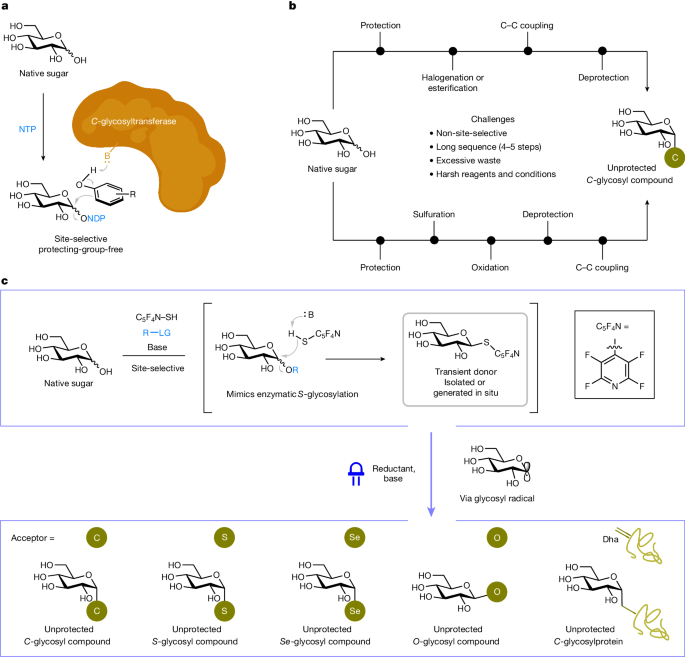
Naturally occurring (native) sugars and carbohydrates contain numerous hydroxyl groups of similar reactivity1,2. Chemists, therefore, rely typically on laborious, multi-step protecting-group strategies3 to convert these renewable feedstocks into reagents (glycosyl donors) to make glycans. The direct transformation of native sugars to complex saccharides remains a notable challenge. Here we describe a photoinduced approach to achieve site- and stereoselective chemical glycosylation from widely available native sugar building blocks, which through homolytic (one-electron) chemistry bypasses unnecessary hydroxyl group masking and manipulation. This process is reminiscent of nature in its regiocontrolled generation of a transient glycosyl donor, followed by radical-based cross-coupling with electrophiles on activation with light. Through selective anomeric functionalization of mono- and oligosaccharides, this protecting-group-free ‘cap and glycosylate’ approach offers straightforward access to a wide array of metabolically robust glycosyl compounds. Owing to its biocompatibility, the method was extended to the direct post-translational glycosylation of proteins.