2024-08-12 カリフォルニア大学サンディエゴ校(UCSD)
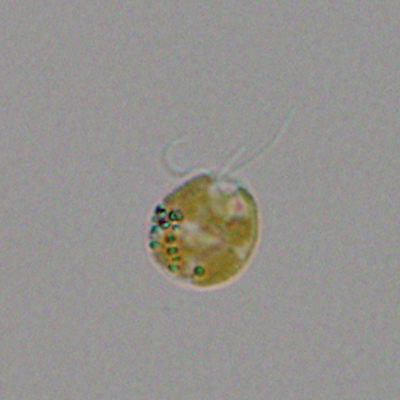
A single cell of the toxin-producing golden alga (Prymnesium parvum). Credit: Greg Southard, Texas Parks and Wildlife Department.
<関連情報>
- https://today.ucsd.edu/story/largest-protein-yet-discovered-builds-algal-toxins
- https://www.science.org/doi/10.1126/science.ado3290
巨大海洋ポリエーテル毒の生合成に関わる巨大ポリケチド合成酵素 Giant polyketide synthase enzymes in the biosynthesis of giant marine polyether toxins
Timothy R. Fallon, Vikram V. Shende, Igor H. Wierzbicki, Amanda L. Pendleton, […], and Bradley S. Moore
Science Published:8 Aug 2024
DOI:https://doi.org/10.1126/science.ado3290
Editor’s summary
Some marine microbes produce exotic organic molecules with varied biological functions. These molecules often require large—sometimes massive—enzymes or enzyme complexes for their biosynthesis. Fallon et al. identified genes from a toxic microalga corresponding to polyketide synthase megaenzymes that are among the largest proteins identified to date, one being nearly 5 megadaltons. The authors dubbed these systems PKZILLAs and matched the individual domains to structural features of prymnesin polyketide fish toxins. Understanding the biosynthesis of these marine toxins will aid with tracking harmful algal blooms and also expands our awareness of the limits of large protein synthesis. —Michael A. Funk
Abstract
Prymnesium parvum are harmful haptophyte algae that cause massive environmental fish kills. Their polyketide polyether toxins, the prymnesins, are among the largest nonpolymeric compounds in nature and have biosynthetic origins that have remained enigmatic for more than 40 years. In this work, we report the “PKZILLAs,” massive P. parvum polyketide synthase (PKS) genes that have evaded previous detection. PKZILLA-1 and -2 encode giant protein products of 4.7 and 3.2 megadaltons that have 140 and 99 enzyme domains. Their predicted polyene product matches the proposed pre-prymnesin precursor of the 90-carbon–backbone A-type prymnesins. We further characterize the variant PKZILLA-B1, which is responsible for the shorter B-type analog prymnesin-B1, from P. parvum RCC3426 and thus establish a general model of haptophyte polyether biosynthetic logic. This work expands expectations of genetic and enzymatic size limits in biology.