2024-08-27 カロリンスカ研究所(KI)
<関連情報>
- https://news.ki.se/a-few-samples-reveal-imbalances-in-vaginal-health
- https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-024-01870-5
膣コミュニティの動態を定義する:日々のマイクロバイオームの変遷、月経、バクテリオファージ、および細菌遺伝子の役割 Defining Vaginal Community Dynamics: daily microbiome transitions, the role of menstruation, bacteriophages, and bacterial genes
Luisa W. Hugerth,Maria Christine Krog,Kilian Vomstein,Juan Du,Zahra Bashir,Vilde Kaldhusdal,Emma Fransson,Lars Engstrand,Henriette Svarre Nielsen &Ina Schuppe-Koistinen
Microbiome Published:19 August 2024
DOI:https://doi.org/10.1186/s40168-024-01870-5
Abstract
Background
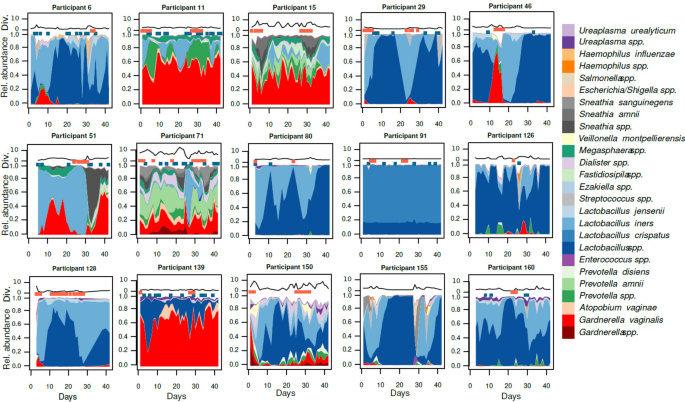
The composition of the vaginal microbiota during the menstrual cycle is dynamic, with some women remaining eu- or dysbiotic and others transitioning between these states. What defines these dynamics, and whether these differences are microbiome-intrinsic or mostly driven by the host is unknown. To address this, we characterized 49 healthy, young women by metagenomic sequencing of daily vaginal swabs during a menstrual cycle. We classified the dynamics of the vaginal microbiome and assessed the impact of host behavior as well as microbiome differences at the species, strain, gene, and phage levels.
Results
Based on the daily shifts in community state types (CSTs) during a menstrual cycle, the vaginal microbiome was classified into four Vaginal Community Dynamics (VCDs) and reported in a classification tool, named VALODY: constant eubiotic, constant dysbiotic, menses-related, and unstable dysbiotic. The abundance of bacteria, phages, and bacterial gene content was compared between the four VCDs. Women with different VCDs showed significant differences in relative phage abundance and bacterial composition even when assigned to the same CST. Women with unstable VCDs had higher phage counts and were more likely dominated by L. iners. Their Gardnerella spp. strains were also more likely to harbor bacteriocin-coding genes.
Conclusions
The VCDs present a novel time series classification that highlights the complexity of varying degrees of vaginal dysbiosis. Knowing the differences in phage gene abundances and the genomic strains present allows a deeper understanding of the initiation and maintenance of permanent dysbiosis. Applying the VCDs to further characterize the different types of microbiome dynamics qualifies the investigation of disease and enables comparisons at individual and population levels. Based on our data, to be able to classify a dysbiotic sample into the accurate VCD, clinicians would need two to three mid-cycle samples and two samples during menses. In the future, it will be important to address whether transient VCDs pose a similar risk profile to persistent dysbiosis with similar clinical outcomes. This framework may aid interdisciplinary translational teams in deciphering the role of the vaginal microbiome in women’s health and reproduction.