2024-12-16 カリフォルニア大学サンディエゴ校(UCSD)
<関連情報>
- https://today.ucsd.edu/story/staphylococcus-aureus-thwarts-vaccines-by-turning-on-a-protein-that-halts-immune-response
- https://www.jci.org/articles/view/179563
- https://www.nature.com/articles/s41467-024-54644-w
- https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(22)00311-0
IL-10を介した病原体駆動型抗体シアル化がワクチン接種を弱体化させる Pathobiont-driven antibody sialylation through IL-10 undermines vaccination
Chih-Ming Tsai, Irshad A. Hajam, J.R. Caldera, Austin W.T. Chiang, Cesia Gonzalez, Xin Du, Biswa Choudhruy, Haining Li, Emi Suzuki, Fatemeh Askarian, Ty’Tianna Clark, Brian Lin, Igor H. Wierzbicki, Angelica M. Riestra, Douglas J. Conrad, David J. Gonzalez, Victor Nizet,, Nathan E. Lewis, and George Y. Liu
Journal of Clinical Investigation Published: December 16, 2024
DOI:https://doi.org/10.1172/JCI179563

Abstract
The pathobiont Staphylococcus aureus (Sa) induces nonprotective antibody imprints that underlie ineffective staphylococcal vaccination. However, the mechanism by which Sa modifies antibody activity is not clear. Herein, we demonstrate that IL-10 is the decisive factor that abrogates antibody protection in mice. Sa-induced B10 cells drive antigen-specific vaccine suppression that affects both recalled and de novo developed B cells. Released IL-10 promotes STAT3 binding upstream of the gene encoding sialyltransferase ST3gal4 and increases its expression by B cells, leading to hyper-α2,3sialylation of antibodies and loss of protective activity. IL-10 enhances α2,3sialylation on cell-wall–associated IsdB, IsdA, and MntC antibodies along with suppression of the respective Sa vaccines. Consistent with mouse findings, human anti-Sa antibodies as well as anti-pseudomonal antibodies from cystic fibrosis subjects (high IL-10) are hypersialylated, compared with anti–Streptococcus pyogenes and pseudomonal antibodies from normal individuals. Overall, we demonstrate a pathobiont-centric mechanism that modulates antibody glycosylation through IL-10, leading to loss of staphylococcal vaccine efficacy.
病原体によって誘導された抑制的免疫インプリントがT細胞ワクチン応答を阻害する Pathobiont-induced suppressive immune imprints thwart T cell vaccine responses
Irshad Ahmed Hajam,Chih-Ming Tsai,Cesia Gonzalez,Juan Raphael Caldera,María Lázaro Díez,Xin Du,April Aralar,Brian Lin,William Duong & George Y. Liu
Nature Communications Published:16 December 2024
DOI:https://doi.org/10.1038/s41467-024-54644-w
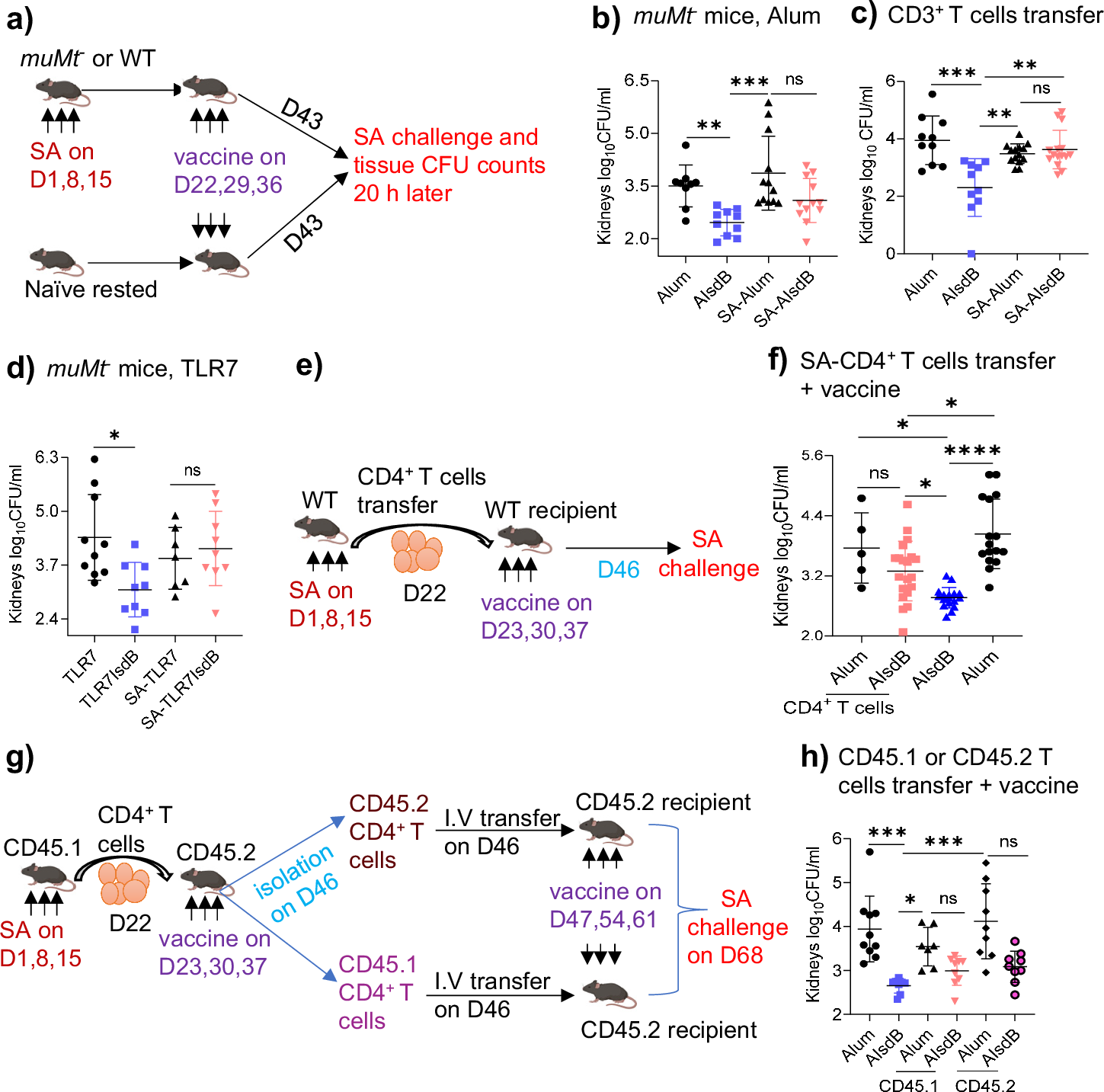
Abstract
Pathobionts have evolved many strategies to coexist with the host, but how immune evasion mechanisms contribute to the difficulty of developing vaccines against pathobionts is unclear. Meanwhile, Staphylococcus aureus (SA) has resisted human vaccine development to date. Here we show that prior SA exposure induces non-protective CD4+ T cell imprints, leading to the blunting of protective IsdB vaccine responses. Mechanistically, these SA-experienced CD4+ T cells express IL-10, which is further amplified by vaccination and impedes vaccine protection by binding with IL-10Rα on CD4+ T cell and inhibit IL-17A production. IL-10 also mediates cross-suppression of IsdB and sdrE multi-antigen vaccine. By contrast, the inefficiency of SA IsdB, IsdA and MntC vaccines can be overcome by co-treatment with adjuvants that promote IL-17A and IFN-γ responses. We thus propose that IL-10 secreting, SA-experienced CD4+ T cell imprints represent a staphylococcal immune escaping mechanism that needs to be taken into consideration for future vaccine development.
黄色ブドウ球菌IsdBワクチンの失敗の根底に非保護的な免疫刷り込みがある Non-protective immune imprint underlies failure of Staphylococcus aureus IsdB vaccine
Chih-Ming Tsai∙ J.R. Caldera∙ Irshad A. Hajam∙ … ∙ Moshe Arditi∙ Nathan E. Lewis∙ George Y. Liu
Cell Host & Microbe Published:July 7, 2022
DOI:https://doi.org/10.1016/j.chom.2022.06.006
Graphical abstract
Highlights
•Prior S. aureus exposure makes the otherwise protective IsdB vaccine non-protective
•IsdB vaccine recalls non-protective humoral memory from prior S. aureus infection
•Specific antibody competition further reduces IsdB vaccine efficacy
•Staphylococcal MntC and FhuD2 vaccines are also blunted by prior S. aureus exposure
Summary
Humans frequently encounter Staphylococcus aureus (SA) throughout life. Animal studies have yielded SA candidate vaccines, yet all human SA vaccine trials have failed. One notable vaccine “failure” targeted IsdB, critical for host iron acquisition. We explored a fundamental difference between humans and laboratory animals—natural SA exposure. Recapitulating the failed phase III IsdB vaccine trial, mice previously infected with SA do not mount protective antibody responses to vaccination, unlike naive animals. Non-protective antibodies exhibit increased α2,3 sialylation that blunts opsonophagocytosis and preferentially targets a non-protective IsdB domain. IsdB vaccination of SA-infected mice recalls non-neutralizing humoral responses, further reducing vaccine efficacy through direct antibody competition. IsdB vaccine interference was overcome by immunization against the IsdB heme-binding domain. Purified human IsdB-specific antibodies also blunt IsdB passive immunization, and additional SA vaccines are susceptible to SA pre-exposure. Thus, failed anti-SA immunization trials could be explained by non-protective imprint from prior host-SA interaction.



