2024-12-16 ペンシルベニア州立大学(PennState)
<関連情報>
- https://www.psu.edu/news/eberly-college-science/story/brain-regions-relieve-effects-chronic-stress-mice-differ-based-sex
- https://www.nature.com/articles/s41380-024-02835-8
- https://www.nature.com/articles/s41380-024-02832-x
ストレスに対する脆弱性を回復力に切り替え、慢性ストレス曝露の影響を逆転させる性特異的GABA作動性微小回路 Sex-specific GABAergic microcircuits that switch vulnerability into resilience to stress and reverse the effects of chronic stress exposure
Tong Jiang,Mengyang Feng,Alexander Hutsell & Bernhard Lüscher
Molecular Psychiatry Published:16 November 2024
DOI:https://doi.org/10.1038/s41380-024-02835-8
Abstract
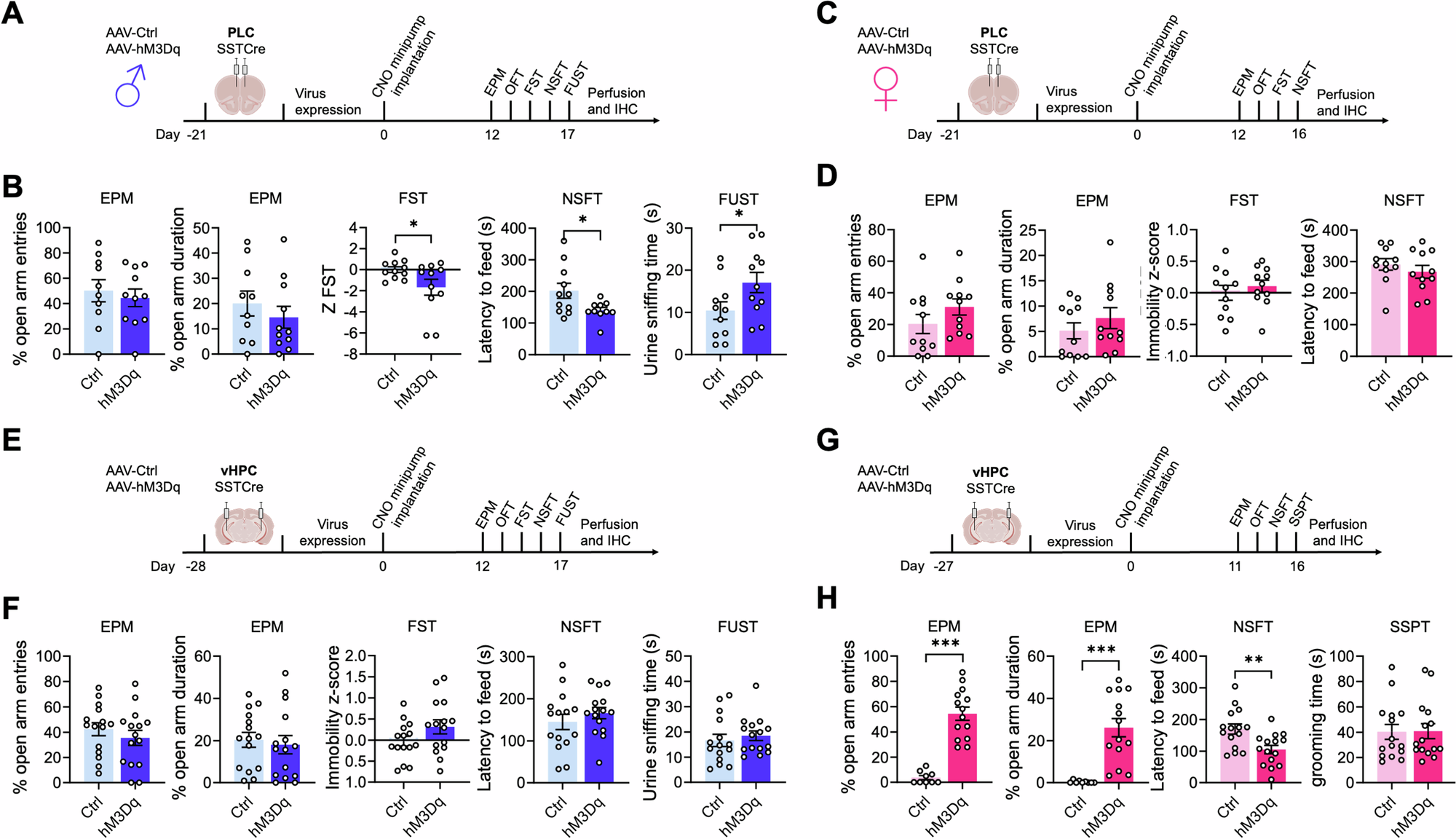
Clinical and preclinical studies have identified somatostatin (SST)-positive interneurons as critical elements that regulate the vulnerability to stress-related psychiatric disorders. Conversely, disinhibition of SST neurons in mice results in resilience to the behavioral effects of chronic stress. Here, we established a low-dose chronic chemogenetic protocol to map these changes in positively and negatively motivated behaviors to specific brain regions. AAV-hM3Dq-mediated chronic activation of SST neurons in the prelimbic cortex (PLC) had antidepressant drug-like effects on anxiety- and anhedonia-like motivated behaviors in male but not female mice. Analogous manipulation of the ventral hippocampus (vHPC) had such effects in female but not male mice. Moreover, the activation of SST neurons in the PLC of male mice and the vHPC of female mice resulted in stress resilience. Activation of SST neurons in the PLC reversed prior chronic stress-induced defects in motivated behavior in males but was ineffective in females. Conversely, activation of SST neurons in the vHPC reversed chronic stress-induced behavioral alterations in females but not males. Quantitation of c-Fos+ and FosB+ neurons in chronic stress-exposed mice revealed that chronic activation of SST neurons leads to a paradoxical increase in pyramidal cell activity. Collectively, these data demonstrate that GABAergic microcircuits driven by dendrite targeting interneurons enable sex- and brain-region-specific neural plasticity that promotes stress resilience and reverses stress-induced anxiety- and anhedonia-like motivated behavior. The data provide a rationale for the lack of antidepressant efficacy of benzodiazepines and superior efficacy of dendrite-targeting, low-potency GABAA receptor agonists, independent of sex and despite striking sex differences in the relevant brain substrates.
慢性ストレス曝露に対するレジリエンスのGABA作動性制御の基礎となる内側前頭前野のトランスクリプトームシグネチャー Transcriptome signatures of the medial prefrontal cortex underlying GABAergic control of resilience to chronic stress exposure
Meiyu Shao,Julia Botvinov,Deepro Banerjee,Santhosh Girirajan & Bernhard Lüscher
Molecular Psychiatry Published:16 November 2024
DOI:https://doi.org/10.1038/s41380-024-02832-x
Abstract
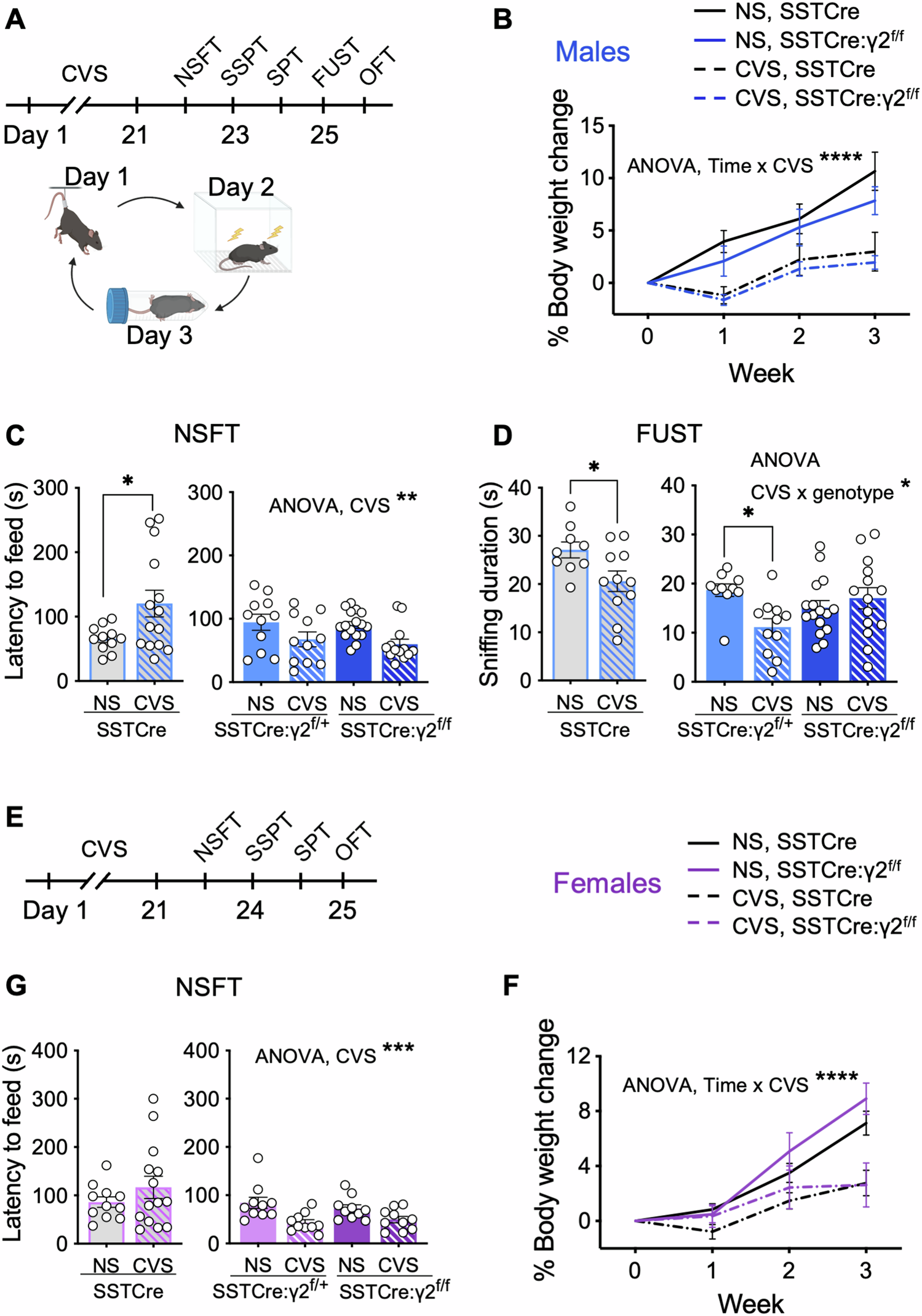
Analyses of postmortem human brains and preclinical studies of rodents have identified somatostatin (SST)-positive, dendrite-targeting GABAergic interneurons as key elements that regulate the vulnerability to stress-related psychiatric disorders. Conversely, genetically induced disinhibition of SST neurons (induced by Cre-mediated deletion of the γ2 GABAA receptor subunit gene selectively from SST neurons, SSTCre:γ2f/f mice) results in stress resilience. Similarly, chronic chemogenetic activation of SST neurons in the medial prefrontal cortex (mPFC) results in stress resilience but only in male and not in female mice. Here, we used RNA sequencing of the mPFC of SSTCre:γ2f/f mice to characterize the transcriptome changes underlying GABAergic control of stress resilience. We found that stress resilience of male but not female SSTCre:γ2f/f mice is characterized by resilience to chronic stress-induced transcriptome changes in the mPFC. Interestingly, the transcriptome of non-stressed SSTCre:γ2f/f (stress-resilient) male mice resembled that of chronic stress-exposed SSTCre (stress-vulnerable) mice. However, the behavior and the serum corticosterone levels of non-stressed SSTCre:γ2f/f mice showed no signs of physiological stress. Most strikingly, chronic stress exposure of SSTCre:γ2f/f mice was associated with an almost complete reversal of their chronic stress-like transcriptome signature, along with pathway changes suggesting stress-induced enhancement of mRNA translation. Behaviorally, the SSTCre:γ2f/f mice were not only resilient to chronic stress-induced anhedonia — they also showed an inversed, anxiolytic-like behavioral response to chronic stress exposure that mirrored the chronic stress-induced reversal of the chronic stress-like transcriptome signature. We conclude that GABAergic dendritic inhibition by SST neurons exerts bidirectional control over behavioral vulnerability and resilience to chronic stress exposure that is mirrored in bidirectional changes in the expression of putative stress resilience genes, through a sex-specific brain substrate.