2025-01-02 カリフォルニア大学サンディエゴ校(UCSD)
<関連情報>
- https://today.ucsd.edu/story/a-ticking-time-bomb-for-liver-cancer
- https://www.nature.com/articles/s41586-024-08317-9
FBP1は老化したMASH肝細胞からの肝癌の進化を制御する FBP1 controls liver cancer evolution from senescent MASH hepatocytes
Li Gu,Yahui Zhu,Shuvro P. Nandi,Maiya Lee,Kosuke Watari,Breanna Bareng,Masafumi Ohira,Yuxiao Liu,Sadatsugu Sakane,Rodrigo Carlessi,Consuelo Sauceda,Debanjan Dhar,Souradipta Ganguly,Mojgan Hosseini,Marcos G. Teneche,Peter D. Adams,David J. Gonzalez,Tatiana Kisseleva,The Liver Cancer Collaborative,Janina E. E. Tirnitz-Parker,M. Celeste Simon,Ludmil B. Alexandrov & Michael Karin
Nature Published:01 January 2025
DOI:https://doi.org/10.1038/s41586-024-08317-9
Abstract
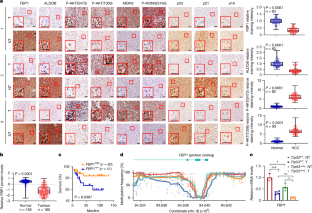
Hepatocellular carcinoma (HCC) originates from differentiated hepatocytes undergoing compensatory proliferation in livers damaged by viruses or metabolic-dysfunction-associated steatohepatitis (MASH)1. While increasing HCC risk2, MASH triggers p53-dependent hepatocyte senescence3, which we found to parallel hypernutrition-induced DNA breaks. How this tumour-suppressive response is bypassed to license oncogenic mutagenesis and enable HCC evolution was previously unclear. Here we identified the gluconeogenic enzyme fructose-1,6-bisphosphatase 1 (FBP1) as a p53 target that is elevated in senescent-like MASH hepatocytes but suppressed through promoter hypermethylation and proteasomal degradation in most human HCCs. FBP1 first declines in metabolically stressed premalignant disease-associated hepatocytes and HCC progenitor cells4,5, paralleling the protumorigenic activation of AKT and NRF2. By accelerating FBP1 and p53 degradation, AKT and NRF2 enhance the proliferation and metabolic activity of previously senescent HCC progenitors. The senescence-reversing and proliferation-supportive NRF2–FBP1–AKT–p53 metabolic switch, operative in mice and humans, also enhances the accumulation of DNA-damage-induced somatic mutations needed for MASH-to-HCC progression.