2025-03-19 ロックフェラー大学
<関連情報>
- https://www.rockefeller.edu/news/37499-new-mechanism-behind-adaptive-immunity-revealed-it-could-impact-how-we-design-vaccines/
- https://www.nature.com/articles/s41586-025-08687-8
超変異の一過性サイレンシングはクローン性バースト時のB細胞親和性を維持する Transient silencing of hypermutation preserves B cell affinity during clonal bursting
Juhee Pae,Niklas Schwan,Bertrand Ottino-Loffler,William S. DeWitt,Amar Garg,Juliana Bortolatto,Ashni A. Vora,Jin-Jie Shen,Alvaro Hobbs,Tiago B. R. Castro,Luka Mesin,Frederick A. Matsen IV,Michael Meyer-Hermann &Gabriel D. Victora
Nature Published:19 March 2025
DOI:https://doi.org/10.1038/s41586-025-08687-8
Abstract
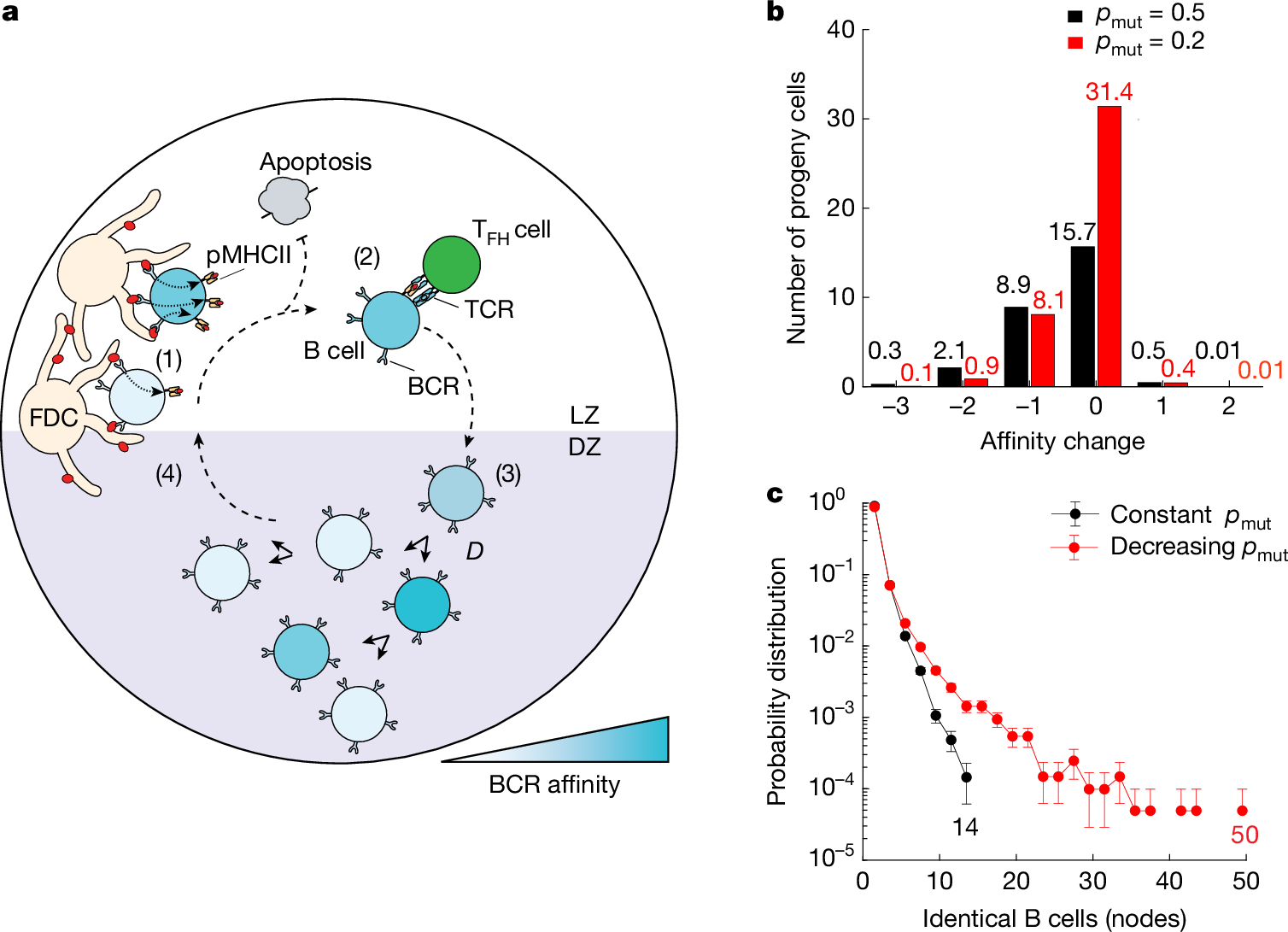
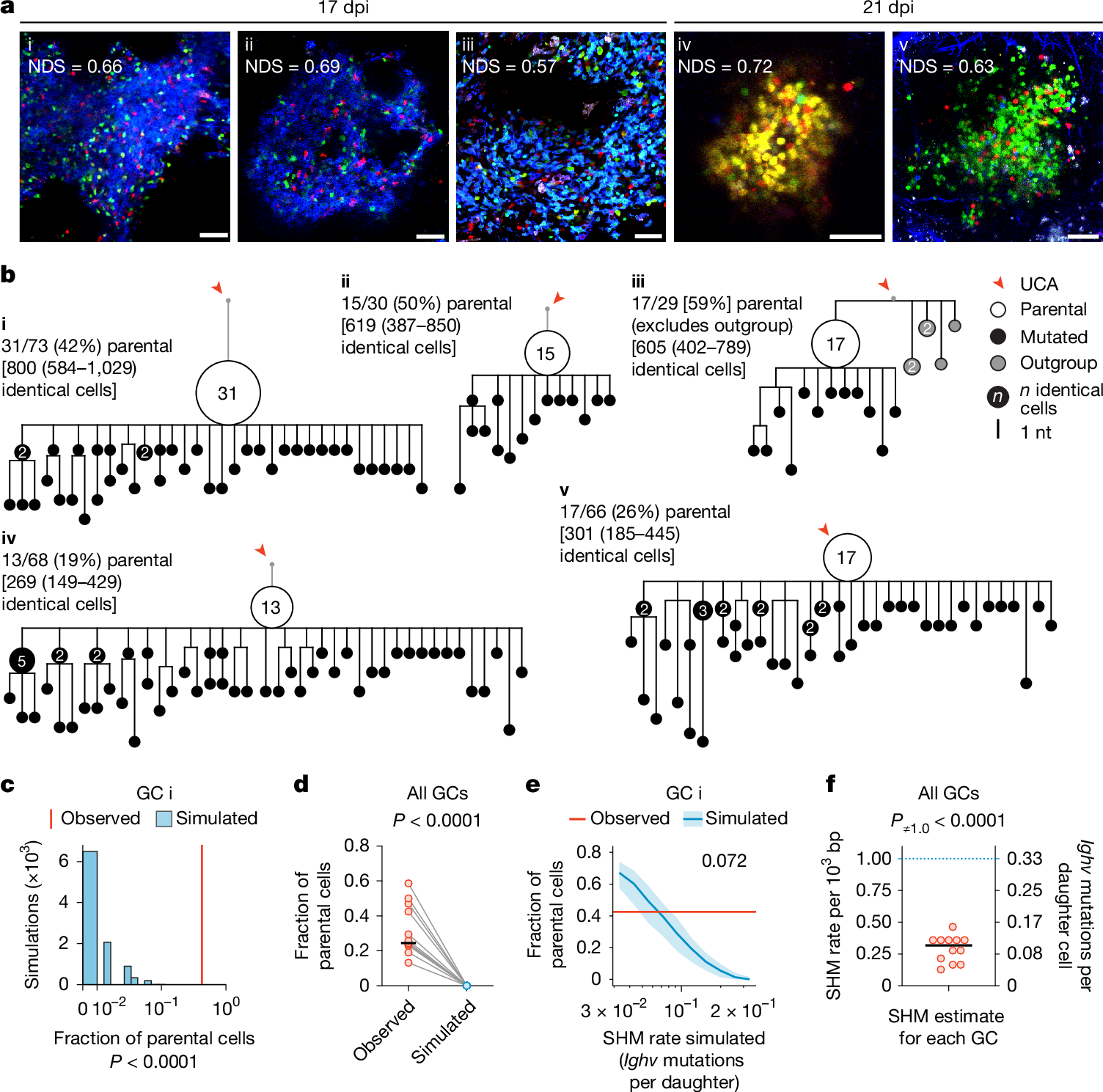
In the course of antibody affinity maturation, germinal centre (GC) B cells mutate their immunoglobulin heavy- and light-chain genes in a process known as somatic hypermutation (SHM). Panels of mutant B cells with different binding affinities for antigens are then selected in a Darwinian manner, which leads to a progressive increase in affinity among the population. As with any Darwinian process, rare gain-of-fitness mutations must be identified and common loss-of-fitness mutations avoided. Progressive acquisition of mutations therefore poses a risk during large proliferative bursts, when GC B cells undergo several cell cycles in the absence of affinity-based selection. Using a combination of in vivo mouse experiments and mathematical modelling, here we show that GCs achieve this balance by strongly suppressing SHM during clonal-burst-type expansion, so that a large fraction of the progeny generated by these bursts does not deviate from their ancestral genotype. Intravital imaging and image-based cell sorting of a mouse strain carrying a reporter of cyclin-dependent kinase 2 (CDK2) activity showed that B cells that are actively undergoing proliferative bursts lack the transient CDK2low ‘G0-like’ phase of the cell cycle in which SHM takes place. We propose a model in which inertially cycling B cells mostly delay SHM until the G0-like phase that follows their final round of division in the GC dark zone, thus maintaining affinity as they clonally expand in the absence of selection.