2025-04-01 カナダ・ブリティッシュコロンビア大学(UBC)
<関連情報>
- https://news.ubc.ca/2025/04/proteomics-reveals-helpful-drug-for-rare-pediatric-cancer/
- https://www.embopress.org/doi/full/10.1038/s44321-025-00212-8
プロテオミクスと個別化PDXモデルにより、進行性悪性腫瘍の治療法を実用的な時間枠内で特定 Proteomics and personalized PDX models identify treatment for a progressive malignancy within an actionable timeframe
Georgina D Barnabas, Tariq A Bhat , Verena Goebeler , Pascal Leclair, Nadine Azzam, Nicole Melong, Colleen Anderson, Alexis Gom, Seohee An, Enes K Ergin, Yaoqing Shen, Agustina Conrrero, Andrew J Mungall , Karen L Mungall, Christopher A Maxwell , Gregor S D Reid , Martin Hirst, Steven Jones , Jennifer A Chan , Donna L Senger , Jason N Berman , Seth J Parker , Jonathan W Bush , Caron Strahlendorf, Rebecca J Deyell , +1 , and Philipp F Lange
EMBO Molecular Medicine Published:1 April 2025
DOI:https://doi.org/10.1038/s44321-025-00212-8
Abstract
Genomics has transformed the diagnostic landscape of pediatric malignancies by identifying and integrating actionable features that refine diagnosis, classification, and treatment. Yet, translating precision oncology data into effective therapies for hard-to-cure childhood, adolescent, and young adult malignancies remains a significant challenge. We present the case for combining proteomics with patient-derived xenograft models to identify personalized treatment for an adolescent with primary and metastatic spindle epithelial tumor with thymus-like elements (SETTLE). Within two weeks of biopsy, proteomics identified elevated SHMT2 as a target for therapy with the anti-depressant sertraline. Drug response was confirmed within two months using a personalized chicken chorioallantoic membrane model of the patient’s SETTLE tumor. Following failure of cytotoxic chemotherapy and second-line therapy, the patient received sertraline treatment and showed decreased tumor growth rates, albeit with clinically progressive disease. We demonstrate that proteomics and fast-track xenograft models provide supportive pre-clinical data in a clinically meaningful timeframe to impact clinical practice. By this, we show that proteome-guided and functional precision oncology are feasible and valuable complements to the current genome-driven precision oncology practices.
Synopsis
A precision medicine workflow integrating proteomics and personalized xenograft models was developed and applied to an adolescent with progressive SETTLE tumor. Target identification combined with pre-clinical therapeutic efficacy was achieved within a meaningful time frame to inform clinical action.
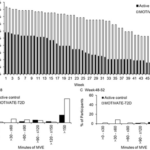
•Proteomic comparison of tumor and normal patient tissue identified elevated SHMT2, involved in one-carbon metabolism, as a putative therapeutic target.
•Combined use of fast track PDX models (chicken chorioallantoic membrane and larval zebrafish) with conventional murine models enabled rapid response modeling while achieving tumor expansion for enhanced tumor biological insights.
•The common anti-depressant and SHMT2 inhibitor, sertraline, was shown to exhibit anti-tumor efficacy against SETTLE PDXs in pre-clinical testing.
•Tumor growth rates was reduced for patient administered sertraline treatment, but without tumor mass reduction that indicated clinically progressive disease.
The paper explained
Problem
Despite significant advances in diagnosis and treatment, cancer continues to be a leading cause of death among children, adolescents, and young adults. Genomics has reshaped the diagnostic landscape of pediatric cancers by identifying and incorporating actionable genetic features that enhance diagnosis, classification, and treatment strategies. However, translating these precision oncology insights into effective therapies for difficult-to-treat childhood, adolescent, and young adult cancers remains a substantial challenge.
Results
We integrate proteomics with patient-derived xenograft models to identify a personalized treatment for an adolescent with primary and metastatic spindle epithelial tumor with thymus-like elements (SETTLE). Proteomics identified elevated SHMT2, a key regulator of one-carbon metabolism, as a potential therapeutic target, leading to treatment with the anti-depressant sertraline. The drug response was validated using a personalized chicken embryo and zebrafish xenograft models of the patient’s SETTLE tumor. After the failure of cytotoxic chemotherapy and second-line therapies, the patient was treated with sertraline, resulting in a slower tumor growth rate, though the disease remained clinically progressive.
Impact
Our study highlights the value of combining proteomics and personalized xenograft models with genomics to improve precision oncology for rare pediatric cancers. It demonstrates how this integrated approach can guide personalized treatment decisions, accelerate therapeutic evaluations, and provide clinically relevant data, potentially transforming medical decision-making and clinical practice.