2025-04-21 ペンシルベニア州立大学(PennState)
<関連情報>
- https://www.psu.edu/news/research/story/can-hormone-therapy-improve-heart-health-menopausal-women
- https://journals.lww.com/greenjournal/fulltext/2025/04000/long_term_changes_to_cardiovascular_biomarkers.1.aspx
女性健康イニシアチブホルモン療法臨床試験におけるホルモン療法後の心血管バイオマーカーの長期的変化 Long-Term Changes to Cardiovascular Biomarkers After Hormone Therapy in the Women’s Health Initiative Hormone Therapy Clinical Trials
Nudy, Matthew MD; Aragaki, Aaron K. MS; Jiang, Xuezhi MD, PhD; Manson, JoAnn E. MD, DrPH; Shadyab, Aladdin H. PhD; Jung, Su Yon PhD; Martin, Lisa W. MD; Wild, Robert A. MD, PhD; Womack, Catherine MD; Mouton, Charles P. MD, MBA; Rossouw, Jacques E. MD; Schnatz, Peter F. DO, MBA
Obstetrics & Gynecology Published:April 2025
DOI:10.1097/AOG.0000000000005862
Abstract
OBJECTIVE:
To assess the long-term changes in cardiovascular biomarkers during the WHI (Women’s Health Initiative) hormone therapy (HT) clinical trials of conjugated equine estrogens (CEE) alone and CEE plus medroxyprogesterone acetate (MPA).
METHODS:
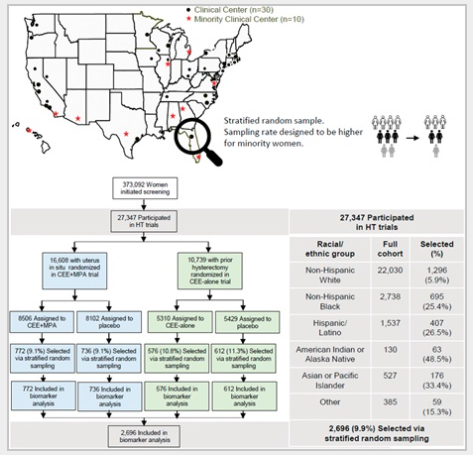
HT trial participants from the CEE alone (n=1,188, 0.625 mg/d CEE or placebo) and the CEE+MPA (n=1,508, 0.625 mg/d CEE plus continuous 2.5 mg/d MPA or placebo) trials provided blood samples at baseline and after 1, 3, and 6 years. Low-density lipoprotein cholesterol (LDL-C; primary endpoint), high-density lipoprotein cholesterol (HDL-C), triglycerides, total cholesterol, lipoprotein(a), glucose, insulin, and homeostatic model assessment for insulin resistance were measured. Repeated-measures regression models estimated the geometric means of each log-transformed biomarker by restricted maximum likelihood. A constant treatment effect across visits was used to estimate the overall effect, expressed as a ratio of geometric means, and was complemented with geometric means (95% CIs) by randomization group and corresponding ratios of geometric means (95% CI; HT vs placebo) at each visit.
RESULTS:
During the intervention phase of the CEE-alone trial, randomization to CEE reduced LDL-C by 11% over 6 years (ratio of geometric means 0.89, 95% CI, 0.88–0.91, P<.001). The overall reduction in LDL-C was similar for CEE+MPA relative to placebo (ratio of geometric means 0.88, 95% CI, 0.86–0.89, P<.001). Relative to placebo, HDL-C and triglycerides were 13.0% and 7.0% higher with CEE and CEE+MPA, respectively. The homeostatic model assessment for insulin resistance decreased by 14.0% and 8.0% for CEE-alone and CEE+MPA trial participants, respectively. Relative to placebo, lipoprotein(a) decreased by 15.0% and 20.0% for participants randomized to CEE alone and CEE+MPA, respectively.
CONCLUSION:
Lipoprotein(a), LDL-C, and homeostatic model assessment for insulin resistance were lower and HDL-C levels were higher for HT compared with placebo. Triglycerides increased in both the CEE and CEE+MPA trials, however. Future research should assess whether other progestogens attenuate the effect of estrogen on HDL-C. These results may be used to counsel younger menopausal women with bothersome symptoms who are deciding whether to initiate oral HT within the context of published effects of oral HT on rates of cardiovascular events.
CLINICAL TRIAL REGISTRATION:
ClinicalTrials.gov, NCT00000611.


