2025-04-23 東京大学

マウス肝臓における飢餓時の代謝トランスオミクスネットワーク
<関連情報>
健常および肥満マウス肝臓における飢餓応答性代謝ネットワークの構造的頑健性と時間的脆弱性 Structural robustness and temporal vulnerability of the starvation-responsive metabolic network in healthy and obese mouse liver
Keigo Morita, Atsushi Hatano, Toshiya Kokaji, Hikaru Sugimoto, […], and Shinya Kuroda
Science Signaling Published:22 Apr 2025
DOI:https://doi.org/10.1126/scisignal.ads2547
Editor’s summary
Obesity perturbs many metabolic processes. Morita et al. constructed a network revealing regulatory relationships between different types of molecules based on omic data collected from the livers of normal and obese mice during food deprivation. The starvation-responsive metabolic network had a similar structure in normal and obese mouse livers. However, the metabolic network in obese mouse livers was wired around different key molecules and showed temporal and pattern disruptions. These defects may help to explain how obesity negates the beneficial effects of intermittent fasting. Together, these results highlight how obesity dysregulates metabolic responses, particularly in the liver, to nutrient deprivation. —Wei Wong
Abstract
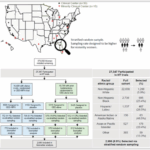
Adaptation to starvation is a multimolecular and temporally ordered process. We sought to elucidate how the healthy liver regulates various molecules in a temporally ordered manner during starvation and how obesity disrupts this process. We used multiomic data collected from the plasma and livers of wild-type and leptin-deficient obese (ob/ob) mice at multiple time points during starvation to construct a starvation-responsive metabolic network that included responsive molecules and their regulatory relationships. Analysis of the network structure showed that in wild-type mice, the key molecules for energy homeostasis, ATP and AMP, acted as hub molecules to regulate various metabolic reactions in the network. Although neither ATP nor AMP was responsive to starvation in ob/ob mice, the structural properties of the network were maintained. In wild-type mice, the molecules in the network were temporally ordered through metabolic processes coordinated by hub molecules, including ATP and AMP, and were positively or negatively coregulated. By contrast, both temporal order and coregulation were disrupted in ob/ob mice. These results suggest that the metabolic network that responds to starvation was structurally robust but temporally disrupted by the obesity-associated loss of responsiveness of the hub molecules. In addition, we propose how obesity alters the response to intermittent fasting.