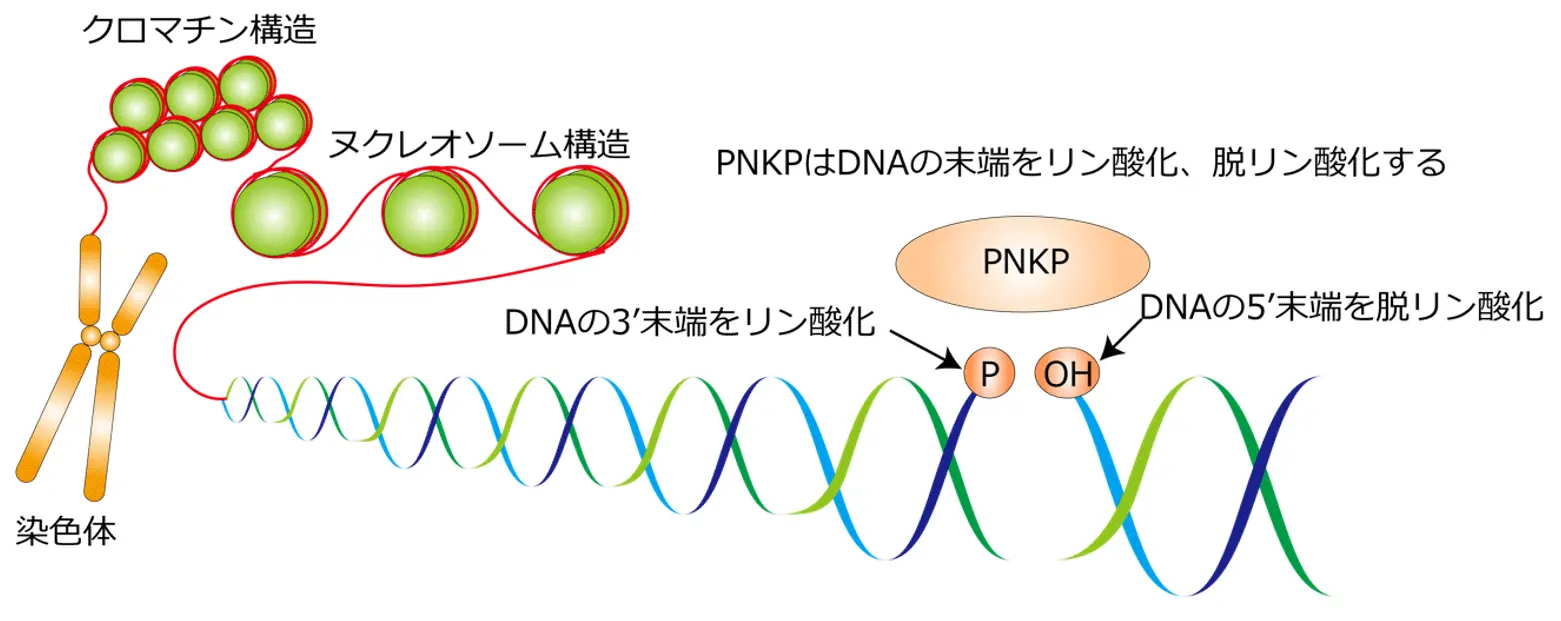
2025-04-23 京都大学
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-04-23
- https://www.kyoto-u.ac.jp/sites/default/files/2025-04/web_2504_Miyakawa-fbcbf71b2e2060886d1fa89b16d51ac1.pdf
- https://www.nature.com/articles/s41477-025-01946-6
Structural insights into CDF1 accumulation on the CONSTANS promoter via a plant-specific DNA-binding domain
Hirotake Furihata,Zhangliang Zhu,Kaisei Nishida,Yasuhito Sakuraba,Akihiro Tsuji,Hayato Yamashita,Shohei Nosaki,Ryo Tachibana,Ayumi Yamagami,Yoshiki Ikeda,Masayuki Abe,Tatsuya Sawasaki,Takeshi Nakano,Shuichi Yanagisawa,Masaru Tanokura & Takuya Miyakawa
Nature Plants Published:22 April 2025
DOI:https://doi.org/10.1038/s41477-025-01946-6
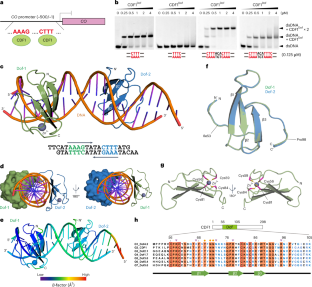
Abstract
DNA-binding with one-finger (Dof) proteins are a family of plant-specific transcription factors distinguished by the highly conserved Dof DNA-binding domain. Various members play crucial roles in diverse plant biological processes. However, it remains unclear how the Dof domain recognizes a restricted set of promoters for gene regulation by binding to just four nucleotides, AAAG/CTTT. Here we present the crystal structure of the Dof domain of CYCLING DOF FACTOR 1 (CDF1), a well-characterized Dof protein acting as a transcriptional repressor by binding to the CONSTANS promoter to regulate photoperiodic flowering, in complex with DNA containing two cis elements. The data reveal that the Dof domain exhibits a unique zinc ribbon fold that includes a three-stranded antiparallel β-sheet and a carboxy-terminal loop, enabling DNA recognition accompanied by directional expansion of the major groove. These features facilitate binding to contiguous target cis elements in a proper arrangement to effectively regulate gene expression.