2025-04-30 東京科学大学
<関連情報>
- https://www.isct.ac.jp/ja/news/nsbyun972v8g
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1370&prevId=&key=01fdae2e1a7c7d5fe45b4b38a8aba61f.pdf
- https://www.sciencedirect.com/science/article/pii/S2589004225007904
光遺伝学を用いたラメリポディア形成におけるアクチン細胞骨格と膜構造の低温電子顕微鏡解析 Cryo-ET of actin cytoskeleton and membrane structure in lamellipodia formation using optogenetics
Hironori Inaba, Tsuyoshi Imasaki, Kazuhiro Aoyama, Shogo Yoshihara, Hiroko Takazaki, Takayuki Kato, Hidemasa Goto, Kaoru Mitsuoka, Ryo Nitta, Takao Nakata
iScience Available online: 24 April 2025
DOI:https://doi.org/10.1016/j.isci.2025.112529
Highlights
- Integrating optogenetics with cryo-ET to visualize cell dynamics at specific times.
- Cryo-ET reveals actin and membrane ultrastructure during lamellipodia formation.
- Mini filopodia act as precursors driving lamellipodia membrane protrusion.
- Actin bundles align parallel to the leading edge during lamellipodia extension.
Summary
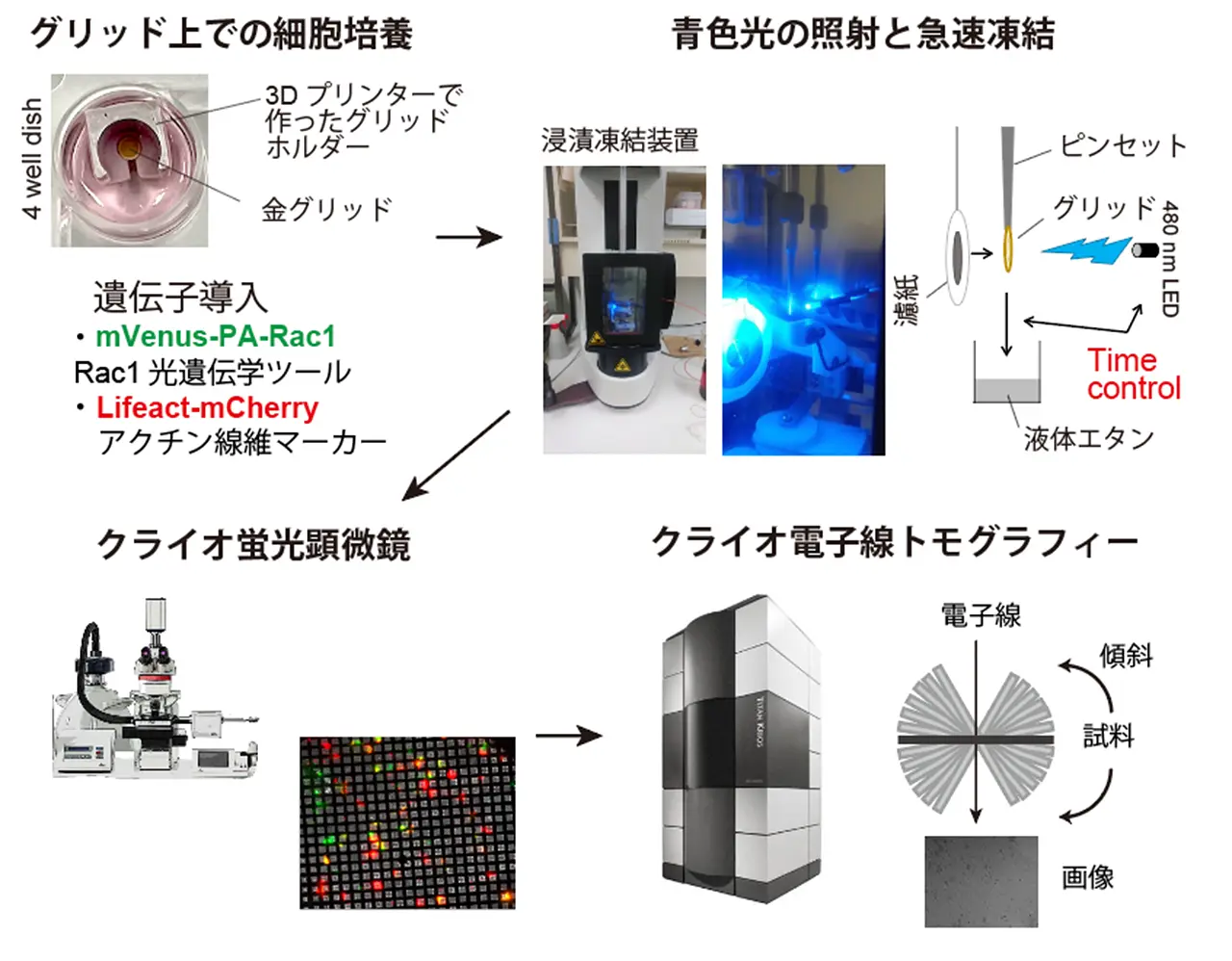
Lamellipodia are sheet-like protrusions essential for cell migration and endocytosis, but their ultrastructural dynamics remain poorly understood because conventional electron microscopy lacks temporal resolution. Here, we combined optogenetics with cryo-electron tomography (cryo-ET) to visualize the actin cytoskeleton and membrane structures during lamellipodia formation with temporal precision. Using photoactivatable-Rac1 (PA-Rac1) in COS-7 cells, we induced lamellipodia formation with a 2-minute blue light irradiation, rapidly vitrified samples, and analyzed their ultrastructure with cryo-ET. We obtained 16 tomograms of lamellipodia at different degrees of extension from three cells. These revealed small protrusions with unbundled actin filaments, “mini filopodia” composed of short, bundled actin filaments at the leading edge, and actin bundles running nearly parallel to the leading edge within inner regions of lamellipodia, suggesting dynamic reorganizations of the actin cytoskeleton. This approach provides a powerful framework for elucidating the ultrastructural dynamics of cellular processes with precise temporal control.