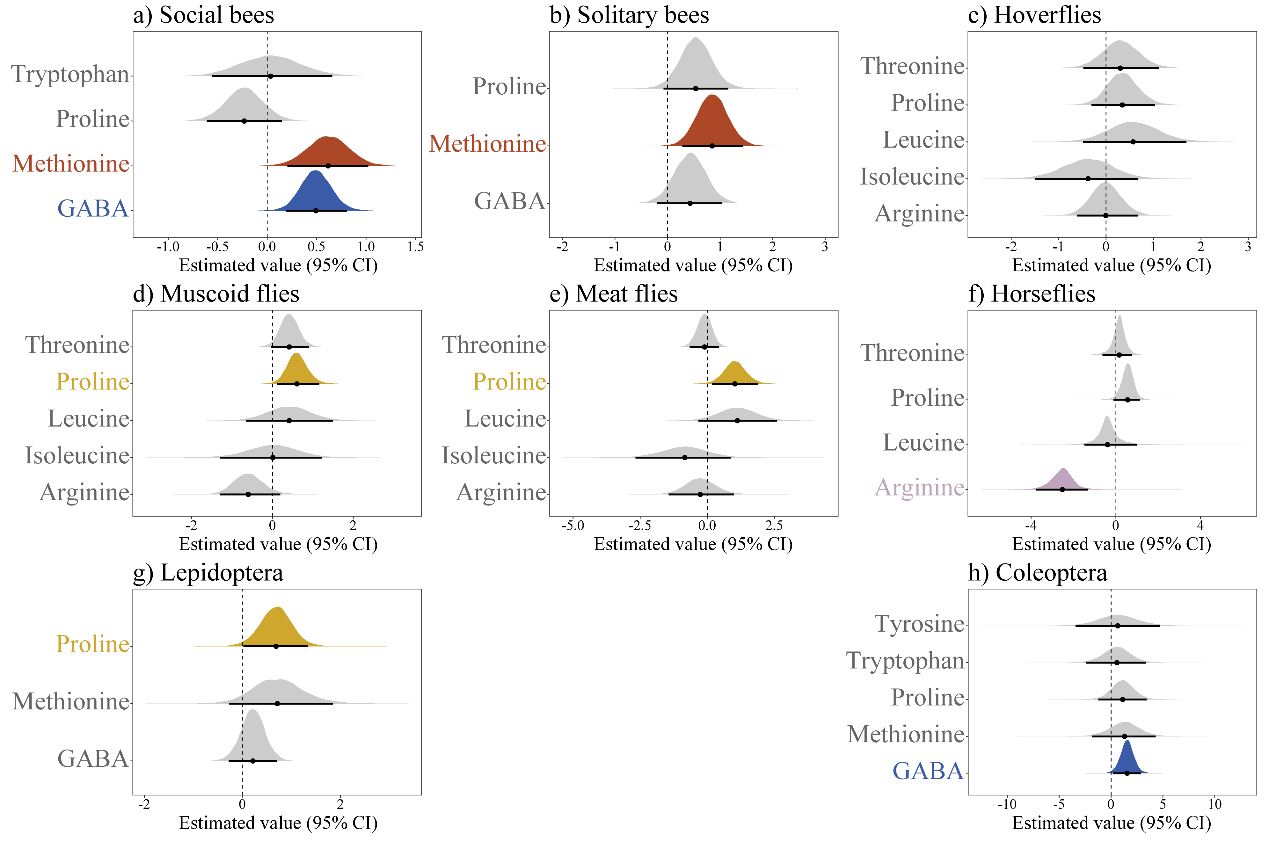
2025-04-27 中国科学院(CAS)
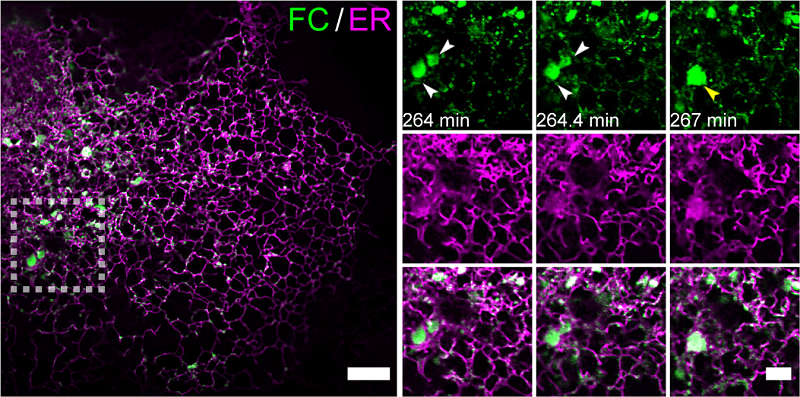
Figure 1. Multi-SIM imaging revealed the accumulation and condensation effects of FC on the endoplasmic reticulum membrane (Image by LI Dong’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202505/t20250506_1042541.shtml
- https://www.cell.com/molecular-cell/fulltext/S1097-2765(25)00304-1
異常な相分離が膜小器官のリモデリングと腫瘍形成を促進する Aberrant phase separation drives membranous organelle remodeling and tumorigenesis
Xinyu Wang ∙ Amin Jiang ∙ Quan Meng ∙ … ∙ Chao Ning ∙ Yajing Lin ∙ Dong Li
Molecular Cell Published:April 23, 2025
DOI:https://doi.org/10.1016/j.molcel.2025.04.001
Highlights
- FUS-CREB3L2 drives ER remodeling to form COPII-like compartments via phase separation
- COPII-like compartments promote RIP of FUS-CREB3L2, releasing its N terminus from ER
- The N-terminus of FUS-CREB3L2 activates LGFMS-specific genes in the nucleus
- Genomic DNA inhibits the phase separation of FUS-CREB3L2
Summary
Membrane remodeling is essential for numerous cellular functions. Although liquid-liquid phase separation (LLPS) of intrinsically disordered region (IDR)-rich proteins could drive dramatic membrane remodeling of artificial giant unilamellar vesicles, it remains elusive whether LLPS-mediated membrane-remodeling functions in live cells and what role it plays in specific bioprocesses. Here, we show that three IDR-rich integral transmembrane fusion proteins (MFPs), generated by chromosomal translocations, can lead to de novo remodeling of their located membranous organelles. Taking FUS-CREB3L2, prevalent in low-grade fibromyxoid sarcoma (LGFMS), as a proof of concept, we recorded super-resolution long-time imaging of endoplasmic reticulum (ER) remodeling dynamics as accumulating FUS-CREB3L2, meanwhile causing spontaneous ER stress to hijack the X-box-binding protein 1 (XBP1) pathway. We further reveal the underlying mechanisms of how FUS-CREB3L2 transduces its tumorigenic signals and aberrant LLPS effects from the ER membrane into the nucleus autonomously, which activates hundreds of LGFMS-specific genes de novo compared with CREB3L2, thus sufficiently reprogramming the cells into an LGFMS-like status.