2025-05-16 北海道⼤学
<関連情報>
- https://www.huhp.hokudai.ac.jp/wp-content/uploads/2025/05/20250516_press.pdf
- https://www.nature.com/articles/s41467-025-59715-0
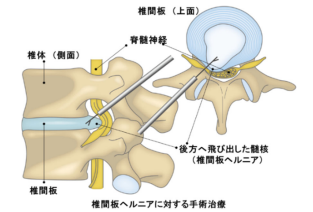
椎間板ヘルニアに対する細胞性生体吸収性超精製アルギン酸ゲル移植術椎間板ヘルニア: フェーズ1/2、非盲検、非ランダム化臨床試験 Acellular, bioresorbable, ultra-purified alginate gel implantation for intervertebral disc herniation: Phase 1/2, open-label, non-randomized clinical trials
Katsuhisa Yamada,Takahiko Hyakumachi,Terufumi Kokabu,Kenichiro Maeda,Toshiyuki Isoe,Khin Khin Tha,Yoichi M. Ito,Takashi Ohnishi,Tsutomu Endo,Daisuke Ukeba,Hiroyuki Tachi,Yuichiro Abe,Yoko Ishikawa,Nozomi Yokota,Takashi Miyakoshi,Osamu Sugita,Norihiro Sato,Norimasa Iwasaki & Hideki Sudo
Nature Communications Published:08 May 2025
DOI:https://doi.org/10.1038/s41467-025-59715-0
Abstract
Discectomy is the current surgical procedure for lumbar intervertebral disc (IVD) herniation. Discectomy was performed to remove the IVD material and relieve the pain inflicted by nerve root compression and axonotoxic effects, such as inflammatory cytokines in the IVD material; however, defects within the IVD caused by discectomy may impair tissue healing and predispose patients to subsequent IVD degeneration. Given that viable cells with the capacity for IVD regeneration are scarce, discectomy alone is not conducive to tissue repair. Here, we report the use of an acellular, bioresorbable, ultra-purified alginate (UPAL) gel implantation system to prevent IVD degeneration after discectomy and demonstrate its feasibility and safety in phase 1/2, open-label, non-randomized clinical trials conducted at a double center. This study comprised two parts: a prospective study on UPAL gel implantation after discectomy in patients with lumbar disc herniation, and a subsequent prospective study on patients who underwent discectomy without UPAL implantation as a control group. The control group was recruited separately. The primary outcomes of this study were the feasibility and safety of UPAL implantation, and the secondary outcomes included physical function scores, self-report questionnaires (SRQs) evaluating pain and health-related quality of life and magnetic resonance imaging (MRI)-based measures of IVD tissues. The UPAL gel implantation demonstrated 100% feasibility and safety (n = 40). The physical function scores improved significantly postoperatively in both groups, with the UPAL group demonstrating greater improvements over time compared to the control group. The SRQ scores were significantly higher in the UPAL group than in the control group from the early postoperative period to 12 weeks. MRI revealed that the disc degeneration score was significantly lower in IVDs with UPAL implantation than in those that underwent discectomy alone. The findings of this study suggest that the UPAL gel is a novel therapeutic strategy after discectomy in cases of lumbar IVD herniation. Trial number: UMIN000034227, UMIN000042282.