2025-06-10 東京科学大学
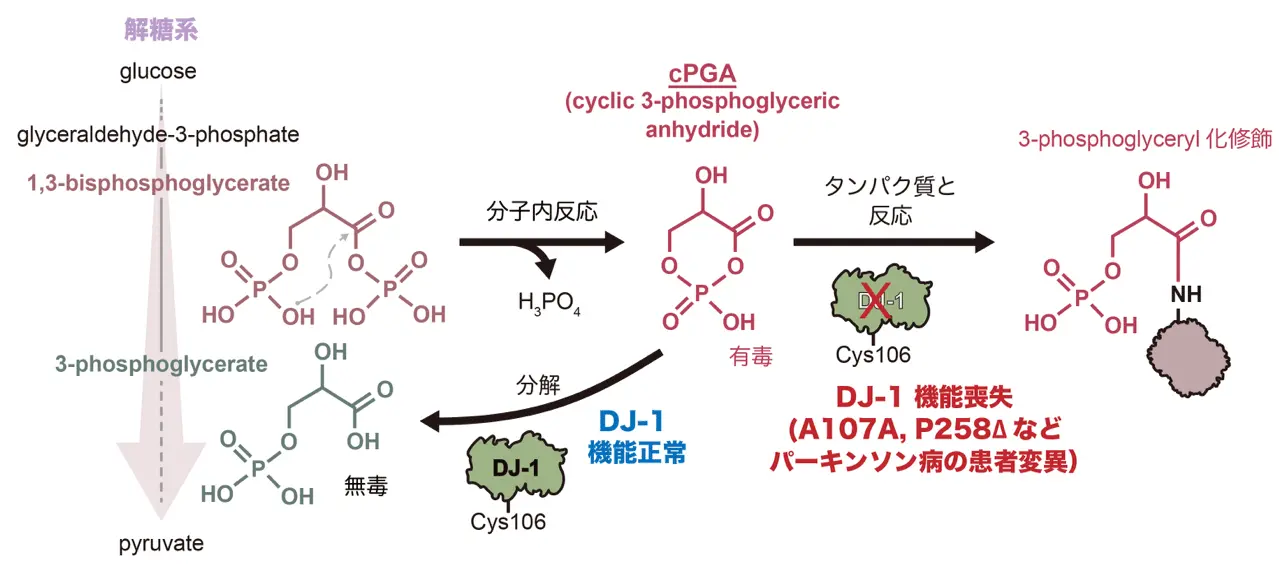 図1. 本研究から解明されたDJ-1の分子機能の概略図
図1. 本研究から解明されたDJ-1の分子機能の概略図
<関連情報>
- https://www.isct.ac.jp/ja/news/gqphj8v1xrbw
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1737&prevId=&key=2d67262c872af8de9c5b9367c39fcfca.pdf
- https://rupress.org/jcb/article/224/8/e202411078/278031/The-reaction-mechanism-for-glycolysis-side-product
パーキンソン病関連DJ-1による解糖副産物分解の反応機構
The reaction mechanism for glycolysis side product degradation by Parkinson’s disease–linked DJ-1
Aiko Watanabe,Shizuka Ogiwara,Mirei Saito,Masaki Mishima,Masahiro Yamashina,Ryuichiro Ishitani,Yutaka Ito,Keiji Tanaka,Fumika Koyano,Koji Yamano,Hidetaka Kosako,Yoshitaka Moriwaki,Noriyuki Matsuda
Journal of Cell Biology Published:June 04 2025
DOI:https://doi.org/10.1083/jcb.202411078
DJ-1/PARK7 is the causative gene for hereditary recessive Parkinson’s disease. Recent studies have reported that DJ-1 hydrolyzes cyclic 3-phosphoglyceric anhydride (cPGA), a highly reactive metabolite. However, the molecular mechanisms underlying cPGA hydrolase activity have yet to be fully elucidated. To gain a more comprehensive understanding of this activity in DJ-1, we performed molecular simulations that predicted how DJ-1 recognizes and hydrolyzes cPGA. The accuracy of these structural predictions was validated through systematic mutational analyses exemplified by loss of activity with the A107P mutation. Although DJ-1 possesses both cPGA hydrolase and α-oxoaldehyde hydratase activities in vitro, we confirmed that DJ-1 dysfunction caused an increase in cPGA-derived modifications but had no effect on α-oxoaldehyde–derived modifications in cells. Importantly, A107 and P158, pathogenic missense mutation sites found in Parkinson’s disease patients, are critical for cPGA hydrolysis both in vitro and in cells. The evidence-based catalytic mechanism for DJ-1 hydrolysis of cPGA that we propose here explains their pathophysiological significance.