2025-05-19 カリフォルニア大学サンフランシスコ校(UCSF)
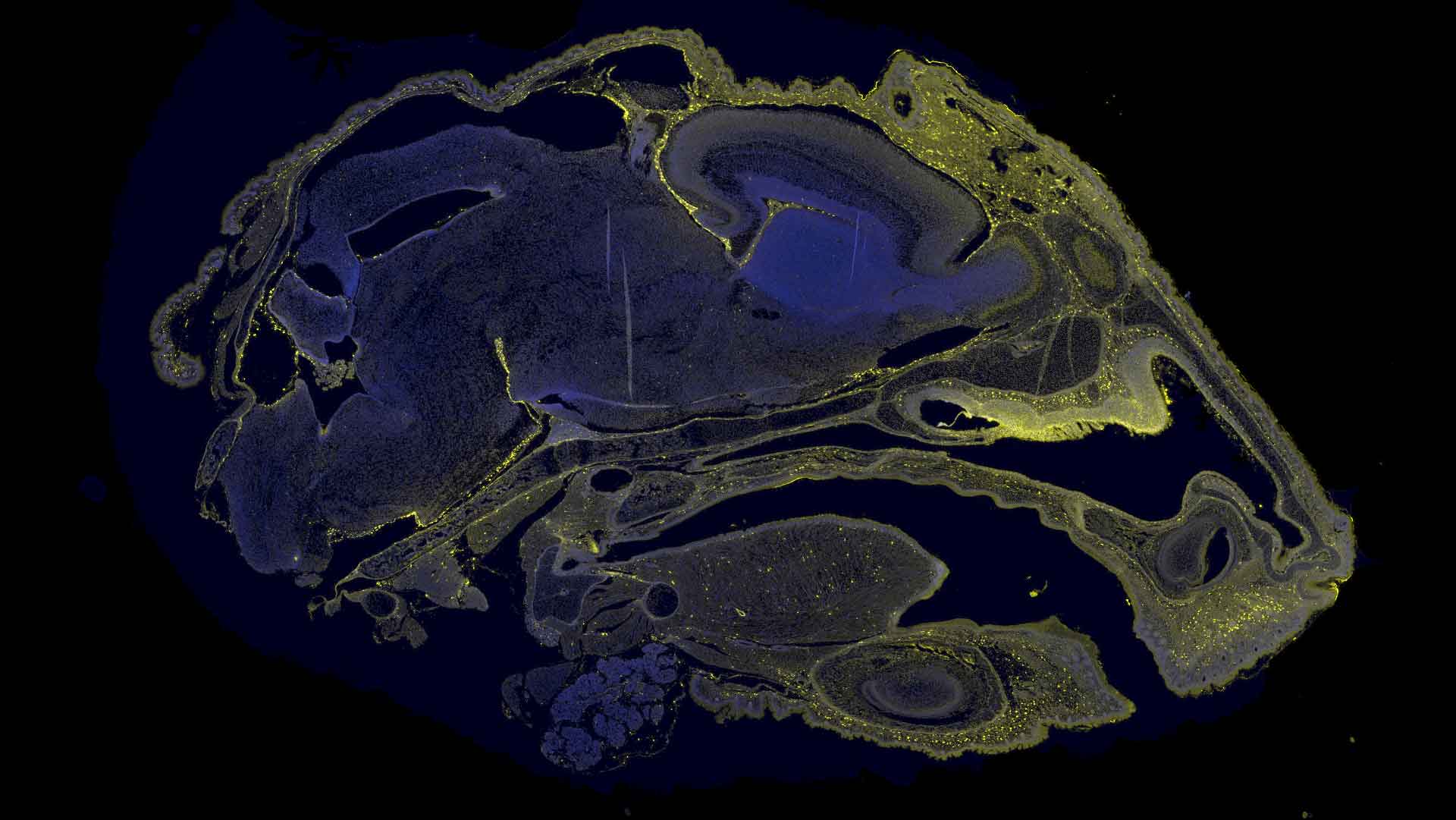
Yellow shows the distribution of the medicine in the mouth, nose, and brain of a fetal mouse after it was injected into the amniotic fluid. Image courtesy of Johns Hopkins University and Ionis
UCSF(カリフォルニア大学サンフランシスコ校)の研究チームは、出生前に重大な遺伝性疾患を治療する革新的手法をマウスとヒツジで実証しました。研究では、脊髄性筋萎縮症(SMA)の治療用アンチセンスオリゴヌクレオチド(ASO)を羊水に注入すると、胎児が飲み込むことで脊髄や脳などへ適切に分布。マウス実験では、出生後の生存率向上、運動機能の維持、運動ニューロン数の保護が確認されました。一方、ヒツジでの注入実験では、安全性が確認され、毒性が認められず、胎児中枢への送達が成功しました。
◆この技術は、羊水を介した非侵襲的・低リスクな経路で、ヒト胎児に医薬品を投与する初の試みに近く、将来的にはアンチセンスRNAや遺伝子治療薬を用いて、鎌状赤血球病や嚢胞性線維症などの重篤な遺伝性疾患を出生前に治療する可能性があります。研究はScience Translational Medicine誌に掲載されました。
<関連情報>
- https://www.ucsf.edu/news/2025/05/429986/step-forward-treating-serious-genetic-disorders-birth
- https://www.science.org/doi/10.1126/scitranslmed.adv4656
羊膜内アンチセンスオリゴヌクレオチド治療は脊髄性筋萎縮症の前臨床モデルにおける表現型を改善する Intra-amniotic antisense oligonucleotide treatment improves phenotypes in preclinical models of spinal muscular atrophy
Beltran Borges, Stephen M. Brown, Wan-Jin Chen, Maria T. Clarke, […] , and Charlotte J. Sumner
Science Translational Medicine Published:14 May 2025
DOI:https://doi.org/10.1126/scitranslmed.adv4656
Editor’s summary
Treatment of spinal muscular atrophy (SMA) ideally starts in the early postnatal period, but patients with severe forms of SMA may still develop substantial neurological deficits despite this. Here, Borges and colleagues showed that prenatal treatment with antisense oligonucleotides (ASOs) via intra-amniotic injection improved outcomes in two mouse models of severe SMA. They also tested the feasibility of intra-amniotic ASO administration in a fetal lamb model, which resulted in broad ASO distribution, including to the central nervous system, although ASO concentrations in different brain regions were expected to be therapeutic in only a subset of animals. These findings highlight the potential utility of intra-amniotic delivery as a route of administration for prenatal treatment for SMA, although further optimization will likely be required before translation to the clinic. —Melissa L. Norton
Abstract
Neurological disorders with onset before or at birth are a leading cause of morbidity and mortality in infants and children. Prenatal treatment has the potential to reduce or prevent irreversible neuronal loss and facilitate normal neurodevelopment. We hypothesized that antisense oligonucleotides (ASOs) delivered to the amniotic fluid by intra-amniotic (IA) injection could safely distribute to the fetal central nervous system (CNS) and provide therapeutic benefit in the motor neuron disease spinal muscular atrophy (SMA), caused by mutations of the survival of motor neuron 1 gene (SMN1), leading to deficiency of SMN protein. Although the splice-switching ASO nusinersen ameliorates SMA when delivered postnatally, substantial deficits can remain in severely affected infants. Here, IA injection of ASOs into two mouse models of severe SMA increased SMN expression in the CNS. In SMAΔ7 mice, which manifest pathology in utero, prenatal treatment improved motor neuron numbers, motor axon development, motor behavioral tests, and survival when compared with those in mice treated postnatally (between P1 and P3). To assess the feasibility of prenatal treatment in a large-animal model, ASOs were delivered midgestation to fetal sheep by IA or intracranial injection. ASOs delivered by IA injection distributed to the spinal cord at therapeutic concentrations and to multiple peripheral tissues without evidence of substantial toxicity to the fetus or mother. These data demonstrated that IA delivery of ASOs holds potential as a minimally invasive approach for prenatal treatment of SMA and possibly other severe, early-onset neurological disorders.