2025-06-09 中国科学院(CAS)
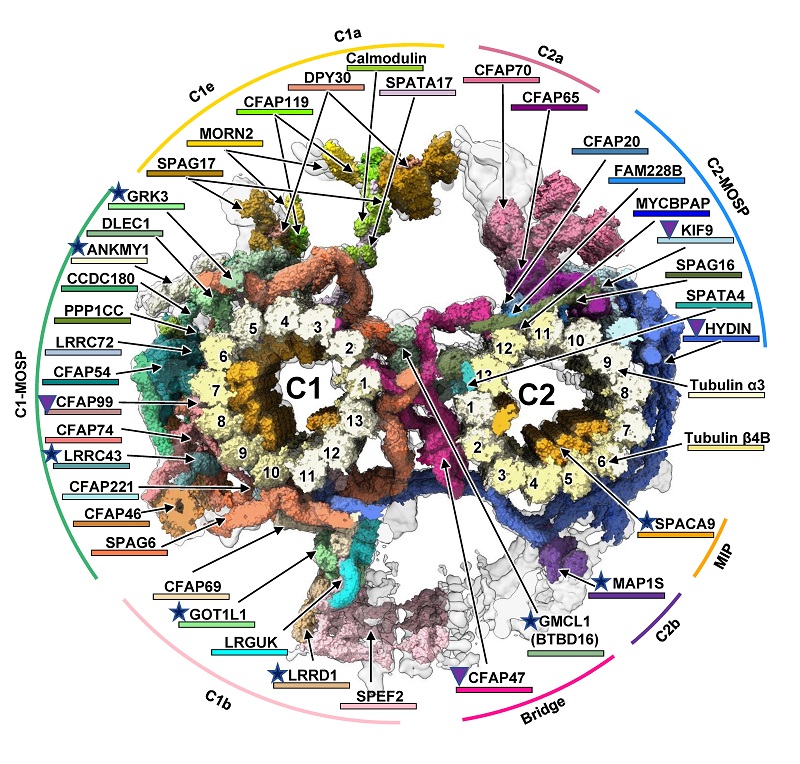 Schematic Diagram of the In Situ Structure of Mouse Sperm Axonemal Central Apparatus (Image by SUN Fei’s group)
Schematic Diagram of the In Situ Structure of Mouse Sperm Axonemal Central Apparatus (Image by SUN Fei’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202506/t20250612_1045479.shtml
- https://www.nature.com/articles/s41422-025-01135-2
マウス精子中枢器官の原位置構造が精子無力症のメカニズムに関する洞察を明らかにする In situ structure of the mouse sperm central apparatus reveals mechanistic insights into asthenozoospermia
Yun Zhu,Tingting Lin,Guoliang Yin,Linhua Tai,Lianwan Chen,Jing Ma,Guoning Huang,Yi Lu,Zhiyong Zhang,Binbin Wang,Suren Chen & Fei Sun
Cell Research Published:05 June 2025
DOI:https://doi.org/10.1038/s41422-025-01135-2
Abstract
The central apparatus (CA) within the sperm axoneme is vital for sperm motility, yet its molecular architecture and functional mechanisms remain incompletely understood. Combining cryo-electron tomography and AlphaFold2, we resolved the in-cell structure of mouse sperm CA at a subnanometer resolution and built a near-complete atomic model. Our analysis identified 39 CA-associated proteins, including eight previously unreported components. By presenting the full-length structures of CFAP47 and HYDIN, we elucidate their molecular roles in tethering the C1 and C2 microtubules within the CA. Specifically, HYDIN forms a semicircular chain that encircles C1 and C2, with its N-terminal half driving the C1–C2 connection and its C-terminal half providing axial support in C2. CFAP47, the core structural component of the bridge, binds C1 through its N-terminal domains, interacts with HYDIN via its central CFAP47-ring, and anchors to C2 through its C-terminal region. The significantly reduced sperm motility and impaired CA structure observed in Cfap47-knockout mice confirmed the important role of CFAP47. Furthermore, genetic analysis of infertile Chinese men with asthenozoospermia identified previously unreported mutations in the CFAP47. The CA structural model elucidates the pathogenic mechanisms of these mutations, establishing a direct link between CFAP47 dysfunction and impaired sperm motility. Therefore, our study provides mechanistic insights into CA-related fertility disorders.