2025-06-17 シカゴ大学(UChicago)
<関連情報>
- https://news.uchicago.edu/story/high-dose-radiation-therapy-may-fuel-cancer-spread-uchicago-study-finds
- https://www.uchicagomedicine.org/forefront/cancer-articles/study-reveals-surprising-side-effects-of-high-dose-radiation-therapy
- https://www.nature.com/articles/s41586-025-08994-0
- https://www.cell.com/cancer-cell/fulltext/S1535-6108(23)00163-0
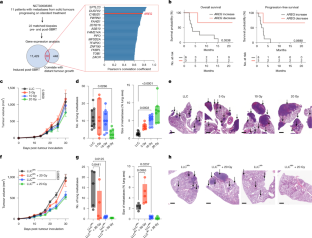
放射線誘発アンフィレグリンは腫瘍転移を促進する Radiation-induced amphiregulin drives tumour metastasis
András Piffkó,Kaiting Yang,Arpit Panda,Janna Heide,Krystyna Tesak,Chuangyu Wen,Katarzyna Zawieracz,Liangliang Wang,Emile Z. Naccasha,Jason Bugno,Yanbin Fu,Dapeng Chen,Leonhard Donle,Ernst Lengyel,Douglas G. Tilley,Matthias Mack,Ronald S. Rock,Steven J. Chmura,Everett E. Vokes,Chuan He,Sean P. Pitroda,Hua Laura Liang & Ralph R. Weichselbaum
Nature Published:14 May 2025
DOI:https://doi.org/10.1038/s41586-025-08994-0
Abstract
The anti-tumour effect of radiotherapy beyond the treatment field—the abscopal effect—has garnered much interest1. However, the potentially deleterious effect of radiation in promoting metastasis is less well studied. Here we show that radiotherapy induces the expression of the EGFR ligand amphiregulin in tumour cells, which reprogrammes EGFR-expressing myeloid cells toward an immunosuppressive phenotype and reduces phagocytosis. This stimulates distant metastasis growth in human patients and in pre-clinical mouse tumour models. The inhibition of these tumour-promoting factors induced by radiotherapy may represent a novel therapeutic strategy to improve patient outcomes.
YTHDF2阻害は放射線治療の抗腫瘍効果を増強する YTHDF2 inhibition potentiates radiotherapy antitumor efficacy
Liangliang Wang ∙ Xiaoyang Dou,, ∙ Shijie Chen ∙ … ∙ Hua Laura Liang ∙ Chuan He ∙ Ralph R. Weichselbaum
Cancer Cell Published:May 25, 2023
DOI:https://doi.org/10.1016/j.ccell.2023.04.019
Highlights
- YTHDF2 elevation in myeloid cells post RT correlates with poor outcome in patients
- YTHDF2 depletion or inhibition in myeloid cells augments antitumor immunity of IR
- YTHDF2 depletion alters MDSC subpopulations in blood and tumors after IR treatment
- The YTHDF2-NF-κB circuit regulates MDSC migration and suppressive function
Summary
RNA N6-methyladenosine (m6A) modification is implicated in cancer progression. However, the impact of m6A on the antitumor effects of radiotherapy and the related mechanisms are unknown. Here we show that ionizing radiation (IR) induces immunosuppressive myeloid-derived suppressor cell (MDSC) expansion and YTHDF2 expression in both murine models and humans. Following IR, loss of Ythdf2 in myeloid cells augments antitumor immunity and overcomes tumor radioresistance by altering MDSC differentiation and inhibiting MDSC infiltration and suppressive function. The remodeling of the landscape of MDSC populations by local IR is reversed by Ythdf2 deficiency. IR-induced YTHDF2 expression relies on NF-κB signaling; YTHDF2 in turn leads to NF-κB activation by directly binding and degrading transcripts encoding negative regulators of NF-κB signaling, resulting in an IR-YTHDF2-NF-κB circuit. Pharmacological inhibition of YTHDF2 overcomes MDSC-induced immunosuppression and improves combined IR and/or anti-PD-L1 treatment. Thus, YTHDF2 is a promising target to improve radiotherapy (RT) and RT/immunotherapy combinations.