2025-06-18 カリフォルニア大学リバーサイド校(UCR)
<関連情報>
- https://news.ucr.edu/articles/2025/06/18/how-common-brain-parasite-disrupts-neural-communication
- https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1012733
神経細胞のトキソプラズマ・ゴンディ感染により細胞外小胞の産生と含量が変化し、アストロサイトの表現型が変化する Toxoplasma gondii infection of neurons alters the production and content of extracellular vesicles directing astrocyte phenotype and contributing to the loss of GLT-1 in the infected brain
Emily Z. Tabaie,Ziting Gao,Nala Kachour,Arzu Ulu,Stacey Gomez,Zoe A. Figueroa,Kristina V. Bergersen,Wenwan Zhong,Emma H. Wilson
PLOS Pathogens Published: June 16, 2025
DOI:https://doi.org/10.1371/journal.ppat.1012733
Abstract
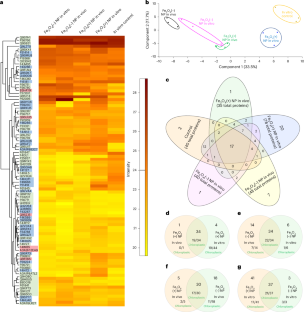
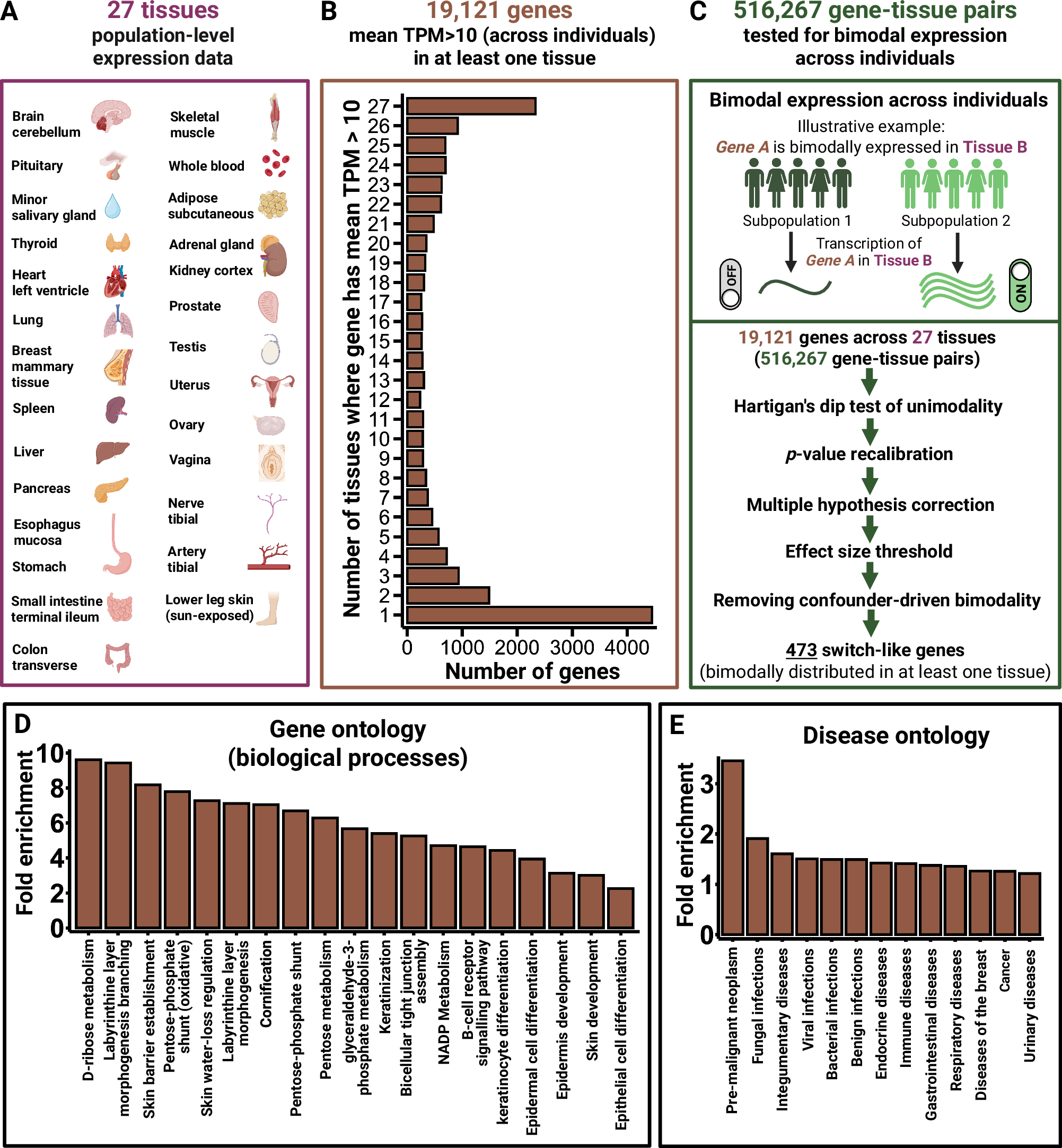
Toxoplasma gondii (T. gondii), a prolific protozoan parasite, forms cysts within neurons of the central nervous system that maintain infection for the lifetime of the host. Astrocytes are fundamental to neuronal health by providing nutrients and structural support and help regulate neurotransmitters by continuous communication with neurons. It is not yet known how infection and the presence of intracellular cysts, disrupts the crucial relationship between these cells. Extracellular vesicles (EVs) function in intracellular communication and can contain proteins, lipids, DNA, miRNA, and other RNA subtypes. EVs are produced by all cells and play an important role in neuronal-astrocyte interactions, including the regulation of glutamate receptors on astrocytes. Previous work has demonstrated that Toxoplasma infection reduces astrocytic expression of the primary glutamate transporter, GLT-1. Here we tested if cyst infection of neurons alters the production and content of EVs. EVs were isolated from uninfected and infected primary murine cortical neurons and their size, concentration, and characterization were confirmed with nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), ELISA, western blot, liquid chromatography (LC)-mass spectrometry (MS)/MS, and microRNA sequencing. Analysis reveals that infection of neurons reduced neuronal production of EVs and altered their protein and miRNA content. In addition to changes in host protein content, EVs from infected neurons contained the Toxoplasma proteins GRA1, GRA2, GRA7, MAG1 and MAG2. Following incubation of neuronal EVs with primary astrocytes, GRA7 protein could be observed within intracellular EVs and the nuclei of GRA7 + EV-containing cells. EVs from infected neurons altered gene expression of astrocytes resulting in an increase in pro-inflammatory transcriptional signatures, along with a downregulation of GLT-1 protein expression with similar transcriptional changes found in astrocytes in vivo. These results demonstrate the ability of a parasitic infection in the brain to alter EV production and the fundamental communication between neurons and astrocytes.
Author summary
Infection with the obligate intracellular parasite, Toxoplasma gondii, leads to neuronal cysts in the brain for the lifetime of the host. Our lab has previously determined that chronic infection leads to loss of astrocytic glutamate transported, GLT-1, leading to neuronal excitotoxicity. GLT-1 can be regulated by neuronal derived extracellular vesicles (EVs). We wanted to determine if cyst infection of neurons altered EV production and content and if EVs derived from cyst-containing neurons changed astrocyte function. Our study found that Toxoplasma cyst infection decreased EV production by neurons and altered EV host protein and miRNA content. In addition, EVs from infected neurons contained parasite derived proteins including the secreted dense granule protein GRA7. Incubation of these EVs with astrocytes led to EV uptake, GRA7 localization to the nucleus, a decrease in GLT-1 expression, and changes in the transcriptional signature of astrocytes to a pro-inflammatory response. Finally, these changes in astrocytic gene expression could be seen in vivo following infection using scRNAseq. This study demonstrates that Toxoplasma cysts alter neuron-astrocyte communication bypassing traditional immune mechanisms of recognition and leading to changes in astrocyte function.