2025-07-01 東京科学大学
<関連情報>
- https://www.isct.ac.jp/ja/news/c0uvyfb051x5
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1849&prevId=&key=3bdca276bb7d79d759276667d674e339.pdf
- https://aacrjournals.org/mcr/article-abstract/doi/10.1158/1541-7786.MCR-24-1095/763220/PAD2-Mediated-Histone-Citrullination-Drives-Tumor?redirectedFrom=fulltext
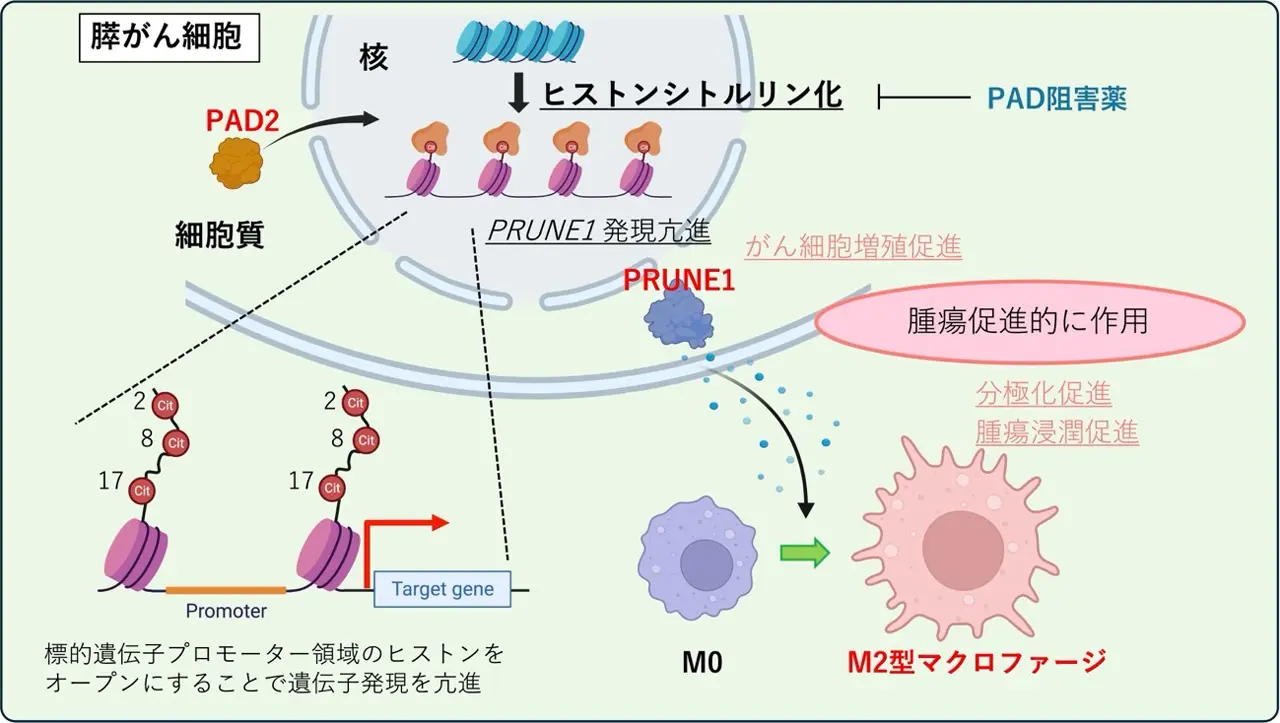
PAD2を介したヒストンシトルリン化が膵臓がんにおける細胞増殖の亢進と微小環境の変化によって腫瘍の進行を促進する PAD2-Mediated Histone Citrullination Drives Tumor Progression by Enhancing Cell Proliferation and Modifying the Microenvironment in Pancreatic Cancer
Kentaro Umemura;Yoshimitsu Akiyama ;Shu Shimada;Megumi Hatano;Ayumi Kono;Koya Yasukawa;Atsushi Kamachi;Yosuke Igarashi;Shu Tsukihara;Yoshiaki Tanji;Koichiro Morimoto;Atsushi Nara;Masahiro Yamane;Keiichi Akahoshi;Hiroaki Ono;Akira Shimizu;Yuji Soejima;Minoru Tanabe;Daisuke Ban;Shinji Tanaka
Molecular Cancer Research Published:June 26 2025
DOI:https://doi.org/10.1158/1541-7786.MCR-24-1095
Abstract
Histone citrullination is catalyzed by peptidyl-arginine deiminases (PAD) that play a role in gene regulation, and several specific inhibitors have been developed. However, the clinical significance, molecular mechanisms of histone citrullination and PADs, and effects of PAD inhibitors in pancreatic ductal adenocarcinoma (PDAC) remain unclear. This study aimed to investigate the role and potential molecular mechanisms of PADs in PDAC. Histone citrullination was upregulated and strongly associated with the nuclear expression of PAD2, one of the PAD family, in human PDAC tissues, correlating with aggressiveness and poor prognosis. PAD2 overexpression increased PDAC cell proliferation, whereas its knockdown had the opposite effect in vitro. PAD2 was recruited to the promoter regions of PRUNE1 and E2F1, resulting in the activation of their mRNA expression via increased histone citrullination and chromatin accessibility. PAD2 overexpression enhanced tumorigenicity and increased PRUNE1 expression and M2 tumor–associated macrophage (M2 TAM) infiltration in vivo. PAD2 inhibitor suppressed the growth and tumorigenicity of PAD2-expressing PDAC mouse models by reducing PRUNE1 expression and M2 macrophage infiltration. Pad2 knockdown and PAD inhibitor treatment showed similar effects in syngeneic mouse models. The triple-high expression of nuclear PAD2, PRUNE1, and the M2 TAM marker CD206 may serve as independent adverse prognostic factors for human PDAC. Conclusively, PAD2-mediated histone citrullination drives PDAC progression by epigenetically regulating downstream target genes and influencing the tumor microenvironment. The PAD2–PRUNE1–M2 TAM axis presents a promising therapeutic target and prognostic indicator for PDAC.