2025-07-09 インペリアル・カレッジ・ロンドン(ICL)
<関連情報>
- https://www.imperial.ac.uk/news/266060/study-reveals-hidden-benefits-weight-loss/
- https://www.nature.com/articles/s41586-025-09233-2
肥満と減量における脂肪ニッチの選択的リモデリング Selective remodelling of the adipose niche in obesity and weight loss
Antonio M. A. Miranda,Liam McAllan,Guianfranco Mazzei,Ivan Andrew,Iona Davies,Meryem Ertugrul,Julia Kenkre,Hiromi Kudo,Joana Carrelha,Bhavik Patel,Sophie Newton,Weihua Zhang,Alice Pollard,Amy Cross,Oliver McCallion,Mikyung Jang,Ka Lok Choi,Scarlett Brown,Yasmin Rasool,Marco Adamo,Mohamed Elkalaawy,Andrew Jenkinson,Borzoueh Mohammadi,Majid Hashemi,… William R. Scott
Nature Published:09 July 2025
DOI:https://doi.org/10.1038/s41586-025-09233-2
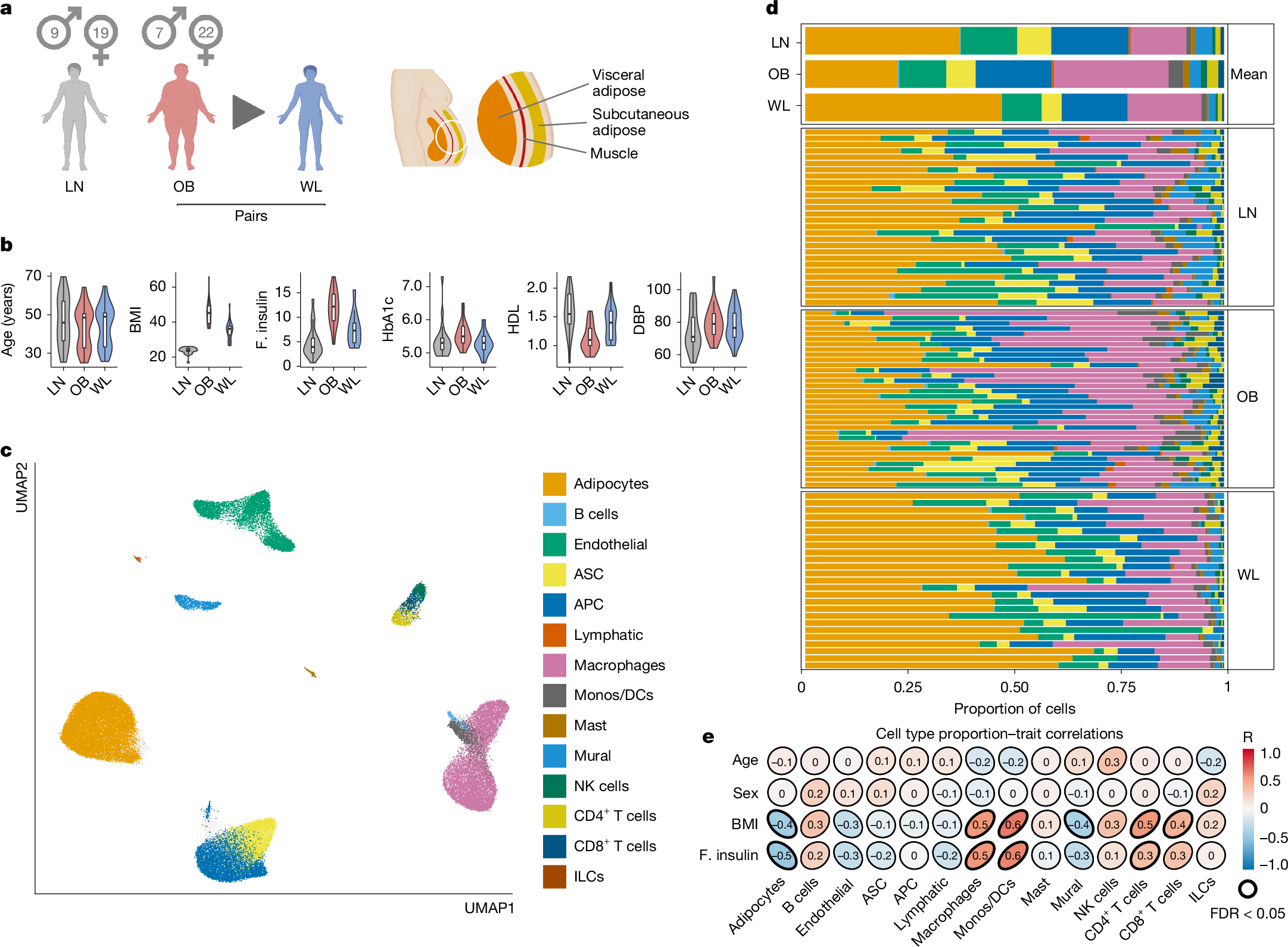
Abstract
Weight loss significantly improves metabolic and cardiovascular health in people with obesity1,2,3. The remodelling of adipose tissue (AT) is central to these varied and important clinical effects4. However, surprisingly little is known about the underlying mechanisms, presenting a barrier to treatment advances. Here we report a spatially resolved single-nucleus atlas (comprising 171,247 cells from 70 people) investigating the cell types, molecular events and regulatory factors that reshape human AT, and thus metabolic health, in obesity and therapeutic weight loss. We discover selective vulnerability to senescence in metabolic, precursor and vascular cells and reveal that senescence is potently reversed by weight loss. We define gene regulatory mechanisms and tissue signals that may drive a degenerative cycle of senescence, tissue injury and metabolic dysfunction. We find that weight loss reduces adipocyte hypertrophy and biomechanical constraint pathways, activating global metabolic flux and bioenergetic substrate cycles that may mediate systemic improvements in metabolic health. In the immune compartment, we demonstrate that weight loss represses obesity-induced macrophage infiltration but does not completely reverse activation, leaving these cells primed to trigger potential weight regain and worsen metabolic dysfunction. Throughout, we map cells to tissue niches to understand the collective determinants of tissue injury and recovery. Overall, our complementary single-nucleus and spatial datasets offer unprecedented insights into the basis of obese AT dysfunction and its reversal by weight loss and are a key resource for mechanistic and therapeutic exploration.