2025-07-22 東北大学
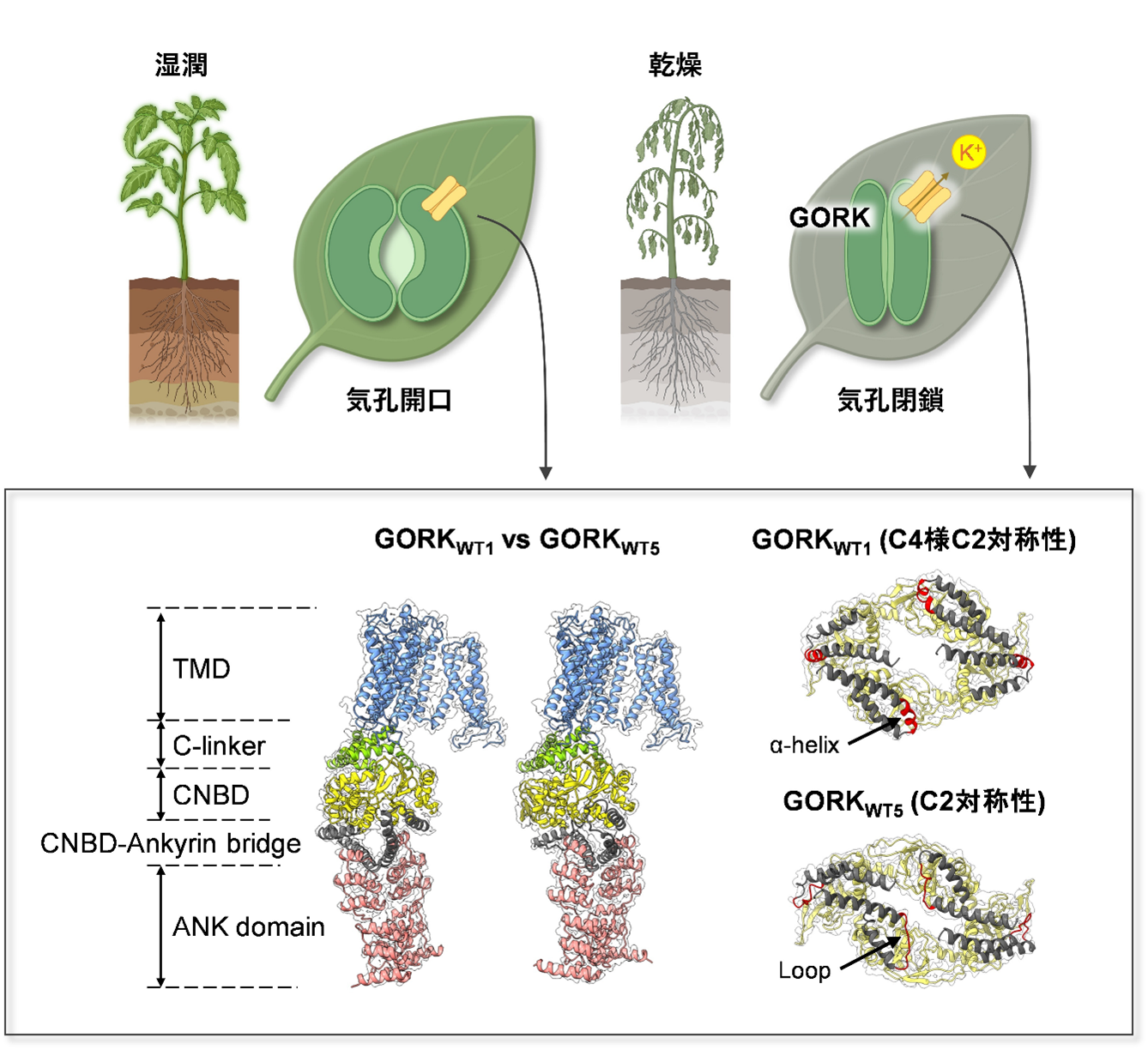
図1.気孔閉鎖を担うK+チャネルGORKの構造。乾燥時や病原菌感染時に、GORKによってK+が細胞外へ排出されると気孔は閉鎖する(図上部)。WT1(Pre-open状態)とWT5(Closed状態)のCNBD-Ankyrin bridge構造を示す(図下部)。
<関連情報>
- https://www.tohoku.ac.jp/japanese/2025/07/press20250722-01-gork.html
- https://www.tohoku.ac.jp/japanese/newimg/pressimg/tohokuuniv-press20250722_01_gork.pdf
- https://www.pnas.org/doi/10.1073/pnas.2500070122
構造解析により、植物の外向性カリウムチャネルGORKの調節メカニズムが、CNBD–アンキリン橋の構造再配置によって制御されていることが明らかになりました Structure reveals a regulation mechanism of plant outward-rectifying K+ channel GORK by structural rearrangements in the CNBD–Ankyrin bridge
Taro Yamanashi, Yuki Muraoka, Tadaomi Furuta, +16 , and Nobuyuki Uozumi
Proceedings of the National Academy of Sciences Published:July 23, 2025
DOI:https://doi.org/10.1073/pnas.2500070122
Significance
The potassium ion efflux channel GORK plays a critical role in closing plant leaf stomata by releasing K+ from guard cells to reduce guard cell volume and turgor. Despite its importance, the regulatory mechanisms controlling GORK activity remain unclear. Here, we have resolved distinct three-dimensional cryo-EM structures of GORK and captured its transition from a tightly closed state to a preopened state. Notably, we show that eight amino acids between the cyclic nucleotide domain and ankyrin domain in the cytosolic C-terminus play a key role in distinguishing the preopened state from other closed states. These findings reveal how structural fluctuations in GORK influence its activity, providing insights into the regulatory mechanisms of GORK-mediated K+ release critical for stomatal function.
Abstract
Guard cells, which regulate stomatal apertures in plants, possess a sophisticated mechanism for regulating turgor pressure. The outward-rectifying “K+out” channel GORK, expressed in guard cells of the plant Arabidopsis thaliana, is a central component that promotes stomatal closure by releasing K+ to the extracellular space, thereby lowering turgor pressure. To date, the structural basis underlying the regulation of the K+ transport activity of GORK is unclear. Using cryo-EM, we determined the structures of the GORK outward-rectifying K+ channel with a resolution of 3.16 to 3.27 Å in five distinct conformations that differ significantly in their C-terminal cyclic nucleotide binding domain (CNBD) and ankyrin repeat (ANK) domain. The C-linker connects the transmembrane domains to the C-terminal domains, i.e., CNBD, CNBD–Ankyrin bridge, and ANK. The structural changes and interactions in the C-linker determine whether the closed state of GORK is closer to the preopen state or in a more removed state from the open state of the channel. In particular, interconversion in the short sequence within the CNBD–Ankyrin bridge plays a decisive role in this determination. This region forms an α-helix in the preopened state, while it adopts a nonhelical structure in further distant closed states. The dynamics of the cytosolic region strongly suggest that the K+ channel activity of GORK is regulated by cytosolic signaling factors during stomatal closure.