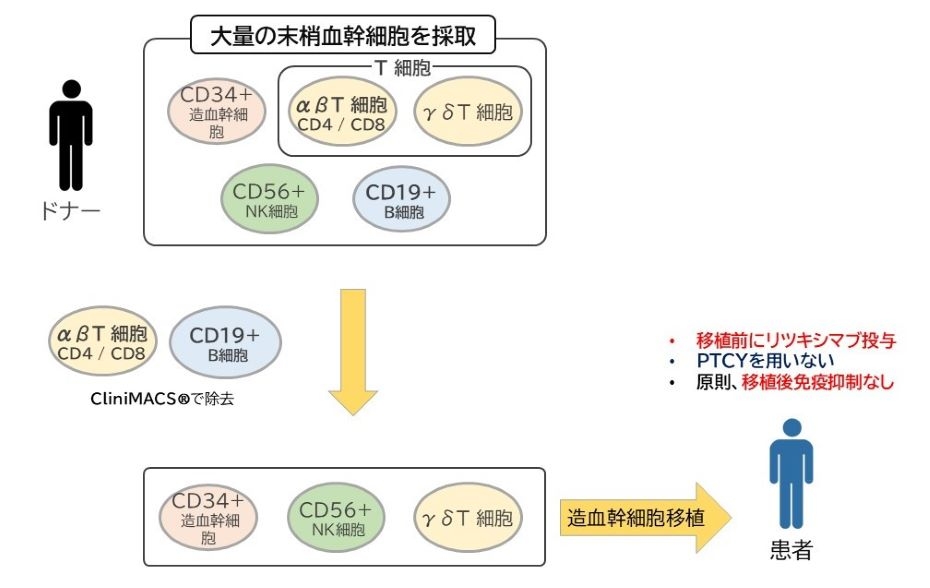
2025-08-12 中国科学院(CAS)
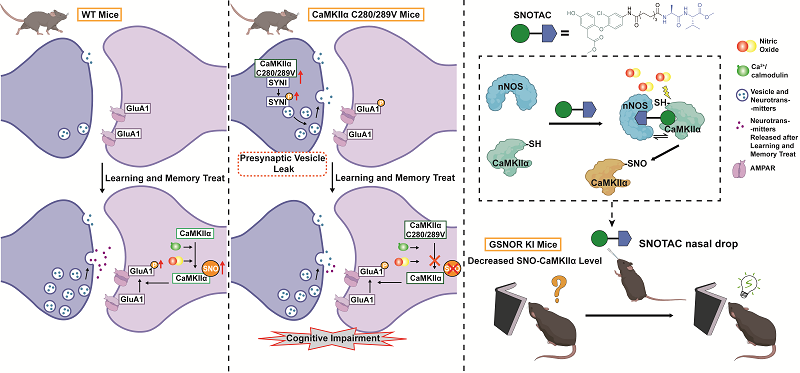
CaMKIIα S-nitrosation and its precise regulation play a key role in learning and memory (Image by CHEN Chang’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202508/t20250820_1051109.shtml
- https://www.sciencedirect.com/science/article/pii/S2213231725002976
CaMKIIαのS-ニトロシル化とそのSNOTACによる精密な酸化還元調節は、学習と記憶に重要な役割を果たす S-nitros(yl)ation of CaMKIIα and its precision redox regulation by SNOTAC plays a critical role in learning and memory
Boyu Chu, Xinhua Qiao, Hui Ye, Xiaoli Cui, Shuli Zhang, Wenting Su, Yuying Zhang, Chuanxin Sun, Xuanhao Wu, Tiepeng Wang, Hua Li, Jianbing Wu, Zhangjian Huang, Chang Chen
Redox Biology Available online: 30 July 2025
DOI:https://doi.org/10.1016/j.redox.2025.103784
Abstract
Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) and nitric oxide (NO) both play vital roles in learning and memory; however, the underlying mechanisms connecting them have remained elusive. To address this question, our study surprisingly observed that during learning and memory tasks, S-nitrosation of CaMKIIα, a key redox-based post-translational modification, significantly increased in mouse hippocampus. We then constructed mice with mutations in the major S-nitrosation sites of CaMKIIα (C280/289V) and found that the mutant mice exhibited remarkable cognitive impairments and attenuated long-term potentiation (LTP). Mechanistically, we demonstrated that the SNO-CaMKIIα mutation increased presynaptic release probability by increasing the interaction and the phosphorylation of synapsin I (Syn1). Excessive vesicle release in the resting state leads to invalid postsynaptic activation, resulting in reduced variability in postsynaptic AMPAR-mediated transmission and impaired response capacity of learning and memory. This reduction of response capacity was also detected in naturally aging mice, indicating it may serve as a determining factor underlying cognitive impairments. Furthermore, we developed the S-nitrosation targeting chimera (SNOTAC), a precision redox modulator designed to enhance the interaction between CaMKIIα and nNOS. Intranasal administration of SNOTAC increased the CaMKIIα S-nitrosation level in mouse hippocampus and successfully rescued learning and memory impairment. These findings establish that redox modification, CaMKIIα S-nitrosation, plays a vital, yet previously unrecognized role in the physiological processes of learning and memory. Moreover, the SNOTAC strategy pioneers a novel paradigm for precision redox intervention, highlighting the potential of targeted redox modulation for cognitive impairment.