2025-09-13 東京科学大学
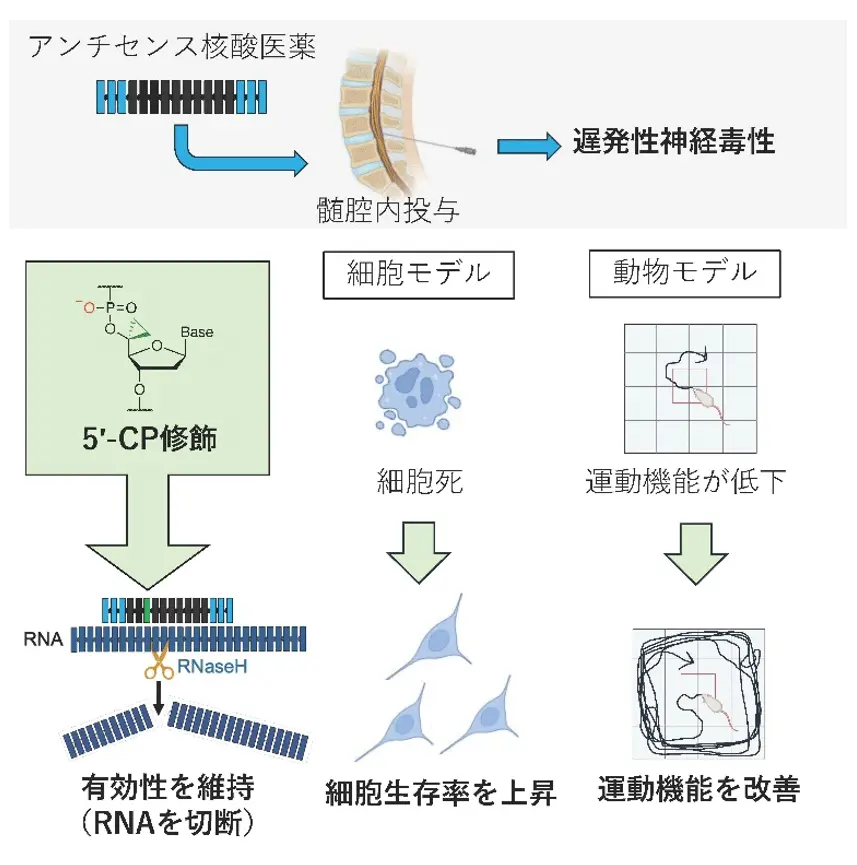
図1.本研究の概要
<関連情報>
- https://www.isct.ac.jp/ja/news/dftg79259aob
- https://www.cell.com/molecular-therapy-family/nucleic-acids/fulltext/S2162-2531(25)00246-X
戦略的化学修飾によるアンチセンスオリゴヌクレオチドの遅発性神経毒性の解明と制御 Unraveling and controlling late-onset neurotoxicity of antisense oligonucleotides through strategic chemical modifications
Takayuki Kuroda ∙ Kotaro Yoshioka ∙ Su Su Lei Mon ∙ … ∙ Takao Yamaguchi ∙ Satoshi Obika ∙ Takanori Yokota
Molecular Therapy – Nucleic Acids Published:September 12, 2025
DOI:https://doi.org/10.1016/j.omtn.2025.102692
Abstract
Antisense oligonucleotides (ASOs) represent an attractive therapeutic approach for CNS disorders. However, ASO-induced neurotoxicity, especially late-onset adverse events, remains a crucial issue, leading to failures in clinical applications. This study aims to determine the neurological features and molecular mechanisms of the late-onset neurotoxicity and provide strategies to overcome this toxicity. We initially established neurobehavioral assays of rodent neurotoxicity with intracerebroventricular and intrathecal injections of various gapmer-type ASOs and a neuronal cytotoxicity analysis. Through both in vitro and in vivo assessments, we identified a site-specific chemical modification, 5′-cyclopropylene (5′-CP), that significantly reduced late-onset neurotoxicity without compromising knockdown activity, providing useful insights into structure-toxicity and structure-activity relationships in ASOs targeting CNS. Additionally, we revealed a toxicity-related mechanism as an elevation of p53-regulated transcripts and paraspeckle protein mislocalization in neuronal cells, which is alleviated through the chemical modifications. Our findings provide mechanistic insights into late-onset ASO-induced neurotoxicity and highlight the potential of optimized chemical modifications to expand the therapeutic window for clinical applications targeting intractable neurological diseases.