2025-10-22 ノースウェスタン大学
<関連情報>
- https://news.northwestern.edu/stories/2025/10/chronic-lung-transplant-rejection-has-been-a-black-box-new-study-gives-answers-drug-targets
- https://insight.jci.org/articles/view/197579
慢性肺移植機能不全の単一細胞解剖により、収束的かつ明確な線維化メカニズムが明らかになった Single-cell dissection of chronic lung allograft dysfunction reveals convergent and distinct fibrotic mechanisms
Yuanqing Yan, Taisuke Kaihou, Emilia Lecuona, Xin Wu, Masahiko Shigemura, Haiying Sun, Chitaru Kurihara, Ruli Gao, Felix L. Nunez-Santana, G.R. Scott Budinger, and Ankit Bharat
JCI Insight Published: October 22, 2025
DOI:https://doi.org/10.1172/jci.insight.197579
Abstract
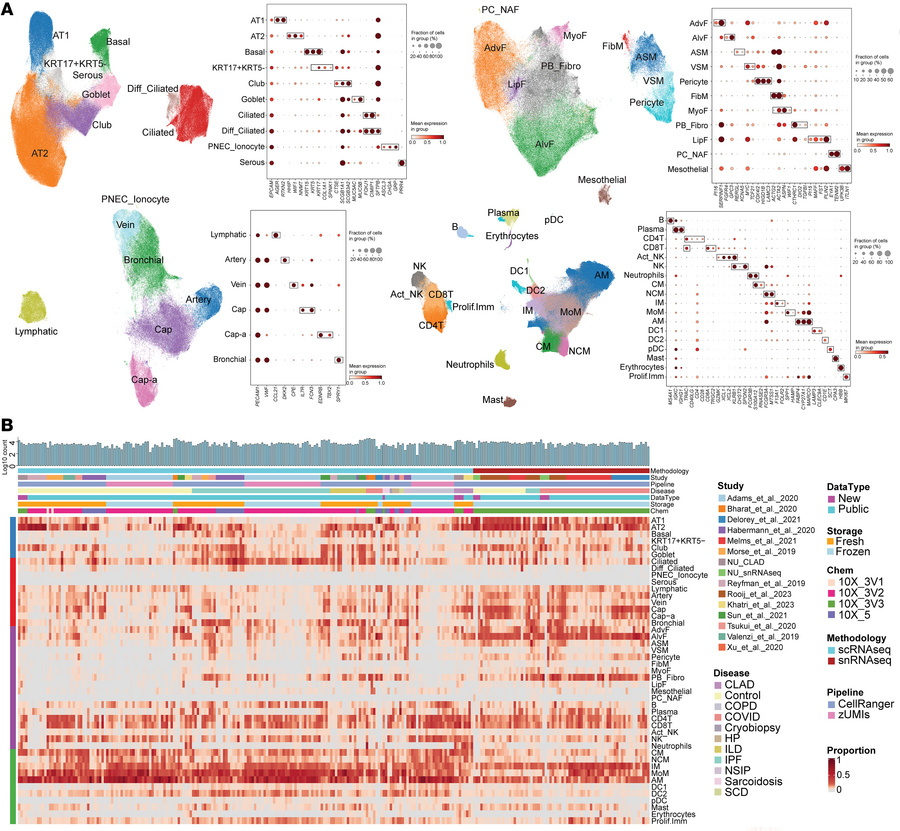
Chronic lung allograft dysfunction (CLAD) is the leading cause of mortality after lung transplantation, yet its molecular mechanisms remain poorly understood. To elucidate the pathogenesis of CLAD, we conducted a comprehensive single-cell transcriptomic analysis of CLAD lungs, integrating our generated datasets with approximately 1.6 million cells from 15 published studies of other fibrotic lung diseases. By applying pseudo-bulk approaches to mitigate batch effects, we identified molecular signatures specific to CLAD and those shared with idiopathic pulmonary fibrosis, COVID-19, and other fibrotic conditions. Our analysis revealed CLAD-specific cellular subsets including Fibro.AT2 cells, exhausted CD8+ T cells, and superactivated macrophages while suggesting that pathogenic keratin 17–positive, keratin 5–negative (KRT17+KRT5–) cells represent a common fibrotic mechanism across fibrotic lung diseases. Additionally, we performed donor-recipient cell deconvolution in lung allografts, uncovering distinct transcriptional programs and intercellular crosstalk between donor- and recipient-derived cells that drive allograft fibrosis. Recipient-derived stromal and immune cells showed enhanced pro-fibrotic and allograft rejection pathways compared with their donor counterparts. By leveraging insights from other fibrotic diseases to elucidate CLAD-specific mechanisms, our study provides a molecular framework for understanding CLAD pathogenesis and identifies potential therapeutic targets for this treatment-refractory condition.