2025-11-07 京都大学
Web要約 の発言:
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-11-07
- https://www.kyoto-u.ac.jp/sites/default/files/2025-11/2511_Clin%26TranslSci_Katada_relj%20web-9cb17891ca3e823ffcd2ed96a0cc10d1.pdf
- https://ascpt.onlinelibrary.wiley.com/doi/10.1111/cts.70317
CYP2C19誘導ボリコナゾール療法:日本人患者における副作用を軽減するための精密医療アプローチ CYP2C19-Guided Voriconazole Therapy: A Precision Medicine Approach to Mitigate Adverse Effects in Japanese Patients
Yoshiki Katada, Daiki Hira, Keisuke Umemura, Yurie Katsube, Hiroki Ishimura, Yusuke Kojima, Machiko Hirai, Moto Kajiwara, Mitsuhiro Sugimoto, Hiroki Endo, Junru Cao, Saki Ohta …
Clinical and Translational Science Published: 18 August 2025
DOI:https://doi.org/10.1111/cts.70317
ABSTRACT
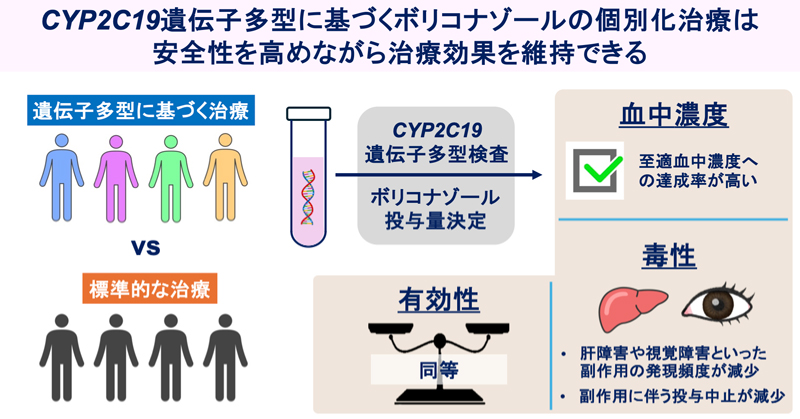
Voriconazole (VRCZ) is a triazole antifungal agent with a broad antifungal spectrum. It is metabolized by hepatic cytochrome P450 (CYP) isozyme CYP2C19, whose genetic polymorphism causes significant variability in drug efficacy and safety. Poor metabolizer alleles of CYP2C19 are more common in Asian populations, increasing the risk of supratherapeutic VRCZ levels. We have developed a novel nomogram based on CYP2C19 genetic polymorphisms. This study aimed to evaluate whether CYP2C19 genotype-guided VRCZ therapy reduces toxicity in Japanese patients. This retrospective study included 64 patients (genotype-guided group, n = 26; comparison group, n = 38). The primary outcome was defined as the composite incidence of adverse events commonly observed with VRCZ, represented by the combined occurrence of grade ≥ 2 hepatotoxicity and visual symptoms. Secondary outcomes included the proportion of patients maintaining VRCZ trough concentrations within the therapeutic range (1–4 μg/mL) and the treatment response at 28 days. The composite incidence of adverse events was significantly lower in the genotype-guided group than in the comparison group (p = 0.003). VRCZ discontinuation due to adverse events occurred in nine patients (23.7%) in the comparison group and one (3.8%) in the genotype-guided group (p = 0.039). More patients in the genotype-guided group were achieved through concentrations within the therapeutic range at the initial sampling point. However, treatment response rates did not differ significantly between the groups. VRCZ administration based on CYP2C19 genotyping improved therapeutic trough levels management and reduced adverse effects while maintaining therapeutic efficacy. These findings highlight the importance of CYP2C19 genotyping for VRCZ treatment in Japanese patients.
Study Highlights
- What is the current knowledge on the topic?
- Voriconazole exposure is highly variable due to CYP2C19 polymorphisms and poor metabolizers, which are common in Asian populations, being prone to toxicity.
- Although TDM is recommended, genotype-guided initial dosing is not routinely implemented in clinical practice in Japan.
- What question did this study address?
- Does initial voriconazole dosing based on CYP2C19 genotype reduce adverse effects and improve therapeutic drug exposure compared to conventional dosing in Japanese patients?
- What does this study add to our knowledge?
- Genotype-guided dosing significantly reduced hepatotoxicity, visual disturbances, and drug discontinuation while improving early attainment of therapeutic trough levels, demonstrating the clinical utility of CYP2C19-guided therapy in populations with high poor metabolizer prevalence.
- How might this change clinical pharmacology or translational science?
- This study supports integrating CYP2C19 genotyping into antifungal stewardship to individualize voriconazole dosing, reduce early toxicity, and improve drug continuation in populations with high frequencies of loss-of-function alleles, advancing precision pharmacotherapy in infectious disease management.